Chromium trioxide
[8] Chromium trioxide is generated by treating sodium dichromate with sulfuric acid:[6] Approximately 100,000 tonnes are produced annually by this or similar routes.
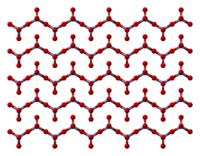
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices.
The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion.
Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications.
Chromium trioxide, being a powerful oxidizer, will ignite organic materials such as alcohols on contact.