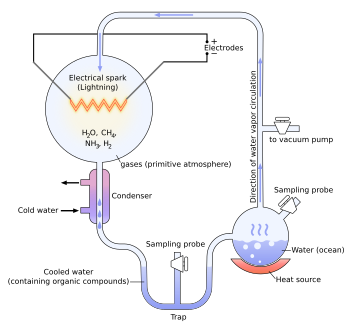
Miller–Urey experiment
B. S. Haldane's hypothesis that the conditions on the primitive Earth favored chemical reactions that synthesized complex organic compounds from simpler inorganic precursors.
[7] Moreover, researchers have shown that transient, hydrogen-rich atmospheres – conducive to Miller-Urey synthesis – would have occurred after large asteroid impacts on early Earth.
[12] While Darwin never publicly wrote about the first organism in his theory of evolution, in a letter to Joseph Dalton Hooker, he speculated:But if (and oh what a big if) we could conceive in some warm little pond with all sorts of ammonia and phosphoric salts, light, heat, electricity etcetera present, that a protein compound was chemically formed, ready to undergo still more complex changes [...]"[13]At this point, it was known that organic molecules could be formed from inorganic starting materials, as Friedrich Wöhler had described Wöhler synthesis of urea from ammonium cyanate in 1828.
[15][17] In 1903, physicist Svante Arrhenius hypothesized that the first microscopic forms of life, driven by the radiation pressure of stars, could have arrived on Earth from space in the panspermia hypothesis.
[18] In the 1920s, Leonard Troland wrote about a primordial enzyme that could have formed by chance in the primitive ocean and catalyzed reactions, and Hermann J. Muller suggested that the formation of a gene with catalytic and autoreplicative properties could have set evolution in motion.
[22] In 1952, Urey postulated that the high temperatures and energies associated with large impacts in Earth's early history would have provided an atmosphere of methane (CH4), water (H2O), ammonia (NH3), and hydrogen (H2), creating the reducing environment necessary for the Oparin-Haldane "primordial soup" scenario.
[23] Stanley Miller arrived at the University of Chicago in 1951 to pursue a PhD under nuclear physicist Edward Teller, another prominent figure in the Manhattan Project.
[24] Miller began to work on how different chemical elements were formed in the early universe, but, after a year of minimal progress, Teller was to leave for California to establish Lawrence Livermore National Laboratory and further nuclear weapons research.
[31] Several energy sources in planetary atmospheres can induce these dissociation reactions and subsequent hydrogen cyanide or aldehyde formation, including lightning,[32] ultraviolet light,[30] and galactic cosmic rays.
[41] The experiments showed that simple organic compounds, including the building blocks of proteins and other macromolecules, can abiotically be formed from gases with the addition of energy.
An article in The New York Times (March 8, 1953) titled "Looking Back Two Billion Years" describes the work of Wollman M. MacNevin at Ohio State University, before the Miller Science paper was published in May 1953.
Wilde's work, published on July 10, 1953, used voltages up to only 600V on a binary mixture of carbon dioxide (CO2) and water in a flow system and did not note any significant reduction products.
[44] According to some, the reports of these experiments explain why Urey was rushing Miller's manuscript through Science and threatening to submit to the Journal of the American Chemical Society.
[7][55] Using high-performance liquid chromatography and mass spectrometry, Bada's lab analyzed old samples from a set of experiments Miller conducted with this apparatus and found some higher yields and a more diverse suite of amino acids.
[7][55] In a separate set of experiments, Miller added hydrogen sulfide (H2S) to the reducing atmosphere, and Bada's analyses of the products suggested order-of-magnitude higher yields, including some amino acids with sulfur moieties.
Geologist William Rubey was one of the first to compile data on gases emitted from modern volcanoes and concluded that they are rich in CO2, H2O, and likely N2, with varying amounts of H2, sulfur dioxide (SO2), and H2S.
[64] A hydrogen-rich prebiotic atmosphere would have large implications for Miller-Urey synthesis in the Hadean and Archean, but later work suggests solutions in that model might have violated conservation of mass and energy.
[66] Taken together, the view that early Earth's atmosphere was weakly reducing, with transient instances of highly-reducing compositions following large impacts is generally supported.
[9][23][60] Conditions similar to those of the Miller–Urey experiments are present in other regions of the Solar System, often substituting ultraviolet light for lightning as the energy source for chemical reactions.
[27] Analysis of the organic fraction of the Murchison meteorite with Fourier-transform ion cyclotron resonance mass spectrometry detected over 10,000 unique compounds,[70] albeit at very low (ppb–ppm) concentrations.
[76] Thus, while creationist arguments focus on the fact that Miller–Urey experiments have not generated all 22 genetically-encoded amino acids,[77] this does not actually conflict with the evolutionary perspective on the origin of life.
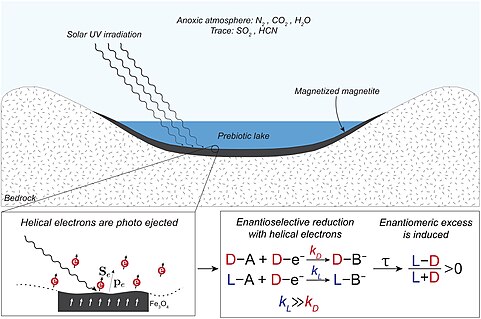
[81] Recent work demonstrates that magnetic mineral surfaces like magnetite can be templates for the enantioselective crystallization of chiral molecules, including RNA precursors, due to the chiral-induced spin selectivity (CISS) effect.
Finally, Miller-Urey and similar experiments primarily deal with the synthesis of monomers; polymerization of these building blocks to form peptides and other more complex structures is the next step of prebiotic chemistry schemes.
[86] Scientists as far back as John Desmond Bernal in the late 1940s thus speculated that clay surfaces would play a large role in abiogenesis, as they might concentrate monomers.