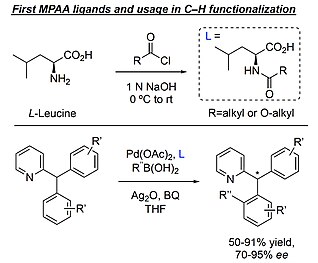
Mono-N-protected amino acids
Mono-N-protected amino acid (MPAA) is a bifunctional ligand that plays a key role in C–H functionalizations by accelerating the reaction rate and imparting specified chirality into the product.
Great strides in the development of MPAA ligands over the past two decades have led to the integral role that enantioselective catalysis now plays in complex organic synthesis.
[9] Initial synthesis occurred by reacting the nucleophilic amino acid in base with a highly electrophilic acyl chloride resulting in one new amide bond formation.
[1] Since the initial discovery, Yu has continued to pioneer the field by expanding the substrate scope, increasing functional group tolerance, and developing ligand variations.
Upon addition of substrate, the N-acyl motif acts as an internal proton acceptor in the concerted metal-deprotonation (CMD) of the transition state for this inner-sphere process.
[14] The highly successful MPAO (mono-protected amino oxazoline) ligand allowed for C(sp3)–H functionalization via arylation of α-methyls, borylation of cyclobutyl carboxylic amides, and boronic cross coupling of alkyl amines.
[20][21] Expanding the reaction substrate scope to non-directed C(sp2)–H bonds, pyridone ligands were developed to functionalize arenes and heteroarenes which proved to be particularly useful in late-state derivatization of bioactive compounds such as estrone, caffeine, and camptothecin.