Sulfur mononitride
Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.
[2] The NS radical is a highly transient species, with a lifetime on the order of milliseconds, but it can be observed spectroscopically over short periods of time through several methods of generation.
NS is too reactive to isolate as a solid or liquid, and has only been prepared as a vapor in low pressure or low-temperature matrices due to its tendency to rapidly oligomerize to more stable, diamagnetic species.
[3] Transmission of electric discharge through a glass tube with quartz windows containing a mixture of nitrogen and sulfur vapor (rigorously free of oxygen) results in the spectrum of emitted light gaining bands consistent with the formation of NS.
[4] Passing a mixture of gaseous N2 and S2Cl2 through the side arm of an absorption cell undergoing microwave discharge produces NS.
[7] The NS radical was detected by LIF spectrum as the product of photolysis of tetranitrogen tetrasulfide (N4S4) gas by a 248 nm laser.
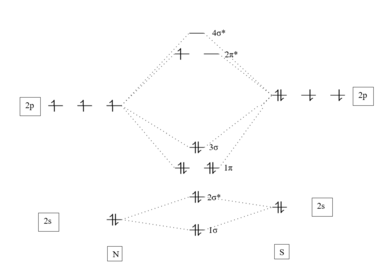
Transition-metal thionitrosyl complexes have been prepared by the following procedures:[12] From X-ray crystallography of many of such metal-thionitrosyl complexes, one can observe that the M-N-S bond angle is nearly linear, suggesting sp hybridization about N. Short M-N distances and long N-S distances reflect the resonance structure of M=N=S having greater contribution than M-N≡S.
[12] Typical v(NS) IR stretching frequencies are approximately 1065 cm−1 for low-valent transition metal complexes and around 1390 cm−1 in the high valent cases, whereas the free gas-phase radical exhibits a 1204 cm−1 signal.
It was found that the large Mulliken spin density remained concentrated on the Fe(NE) core and Fe-N distances experienced little change from the chalcogen atom used.
[3] Molecules in distant astronomical regions can be identified based on their unique rotational transitions, of which the corresponding microwave frequencies are detectable by antennae on Earth.
Measurements conducted with the National Radio Astronomy Observatory telescope at Kitt Peak, Arizona, picked up millimeter-wavelength radiation in Sgr B2 attributed to c-state transitions of NS in the 2Π1/2 state from J=5/2 to J=3/2 at 115.16 GHz.
It's believed that the observed abundance is higher than gas-phase, ion-molecule models due to an unidentified species X-NS photo-dissociating to release NS.
The experimental apparatus to test this involved a primary flame for producing combustion products, which were mixed with NO and SO2 to mimic coal burning byproducts.
1-2% decrease in NOx concentration is observed at various percentages of total fuel inlet (reburn ratio) in the presence of 0.1% SO2, which is attributed to the formation of H2S, HS, and the resulting reaction with NO, giving rise to NS.