Mucic acid
[2] Mucic acid forms a crystalline powder, which melts at 210–230 °C.
[2] Due to the symmetry in the molecule, it is optically inactive even though it has chiral carbon atoms (i.e., it is a meso compound).
[2] The ammonium salt yields on dry distillation carbon dioxide, ammonia, pyrrol and other substances.
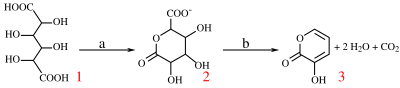
[2] With potassium bisulfate mucic acid forms 3-hydroxy-2-pyrone by dehydration and decarboxylation.
It has been used as a precursor of adipic acid in the way to nylon by a rhenium-catalyzed deoxydehydration reaction.