Nicolaou Taxol total synthesis
[1] Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.
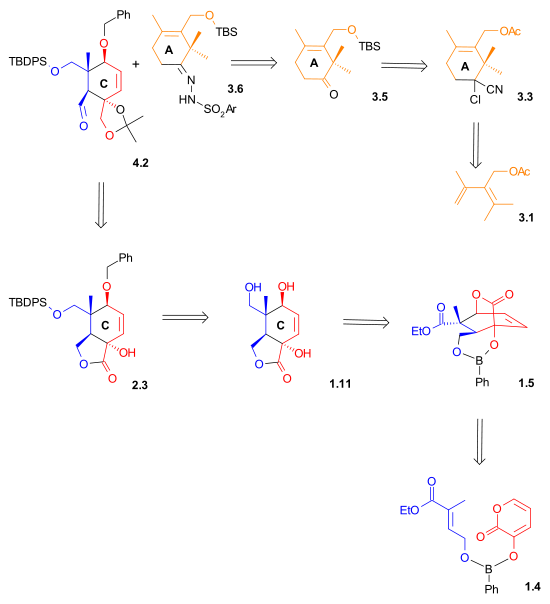
[3][4][5][6] As illustrated in Retrosynthetic Scheme I, Taxol was derived from diol 7.2 by an ester bond formation, according to the Ojima-Holton method.
The A ring synthesis (Scheme 3) started with a Diels-Alder reaction of diene 3.1 with the commercially available dienophile 2-chloroacrylonitrile 3.2 to give cyclohexene 3.3 with complete regioselectivity.
The control of stereochemistry in 4.3 is thought to be derived from the relative hindrance of the Si face in the orientation shown on the right, due to the proximity of the axial methyl group.
The carbonate group also served to create rigidity in the ring structure for the imminent pinacol coupling reaction.
The two silyl ether groups were removed, and diol 4.7 was then oxidized to give dialdehyde 4.8 using N-methylmorpholine N-oxide in the presence of a catalytic amount of tetrapropylammonium perruthenate.
In the final step of the formation of Ring B, a pinacol coupling using conditions developed by McMurry (titanium(III) chloride and a zinc/copper alloy) gave diol 4.9.
The desired enantiomer from resolution, allylic alcohol 5.1 (Scheme 5) was acetylated with acetic anhydride and 4-(dimethylamino)pyridine in methylene chloride to yield monoacetate 5.2.
The Taxol ring (D) was added by an intramolecular nucleophilic substitution involving this hydroxyl group to give oxetane 6.3.
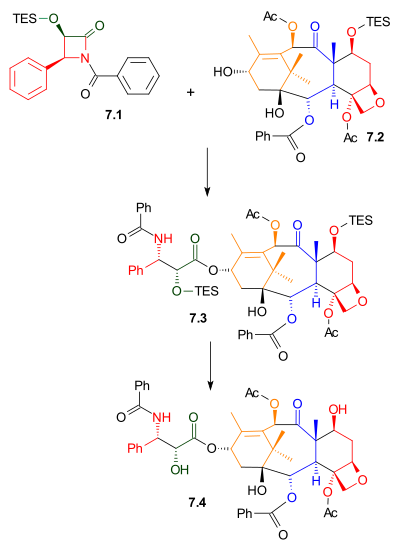
As shown in Scheme 7, Ojima lactam 7.1 reacted with alcohol 7.2 with sodium bis(trimethylsilyl)amide as a base.
The related compound, 10-deacetylbaccatin III, is found in Taxus baccata, also known as the European Yew, in concentrations of 1 gram per kilogram leaves.
A Grignard reaction involving methylmagnesium bromide provided alcohol 4, which was subjected to acid catalyzed elimination to give diene 5.