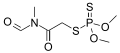
N-Methylformamide
N-Methylformamide (NMF) is a colorless, nearly odorless, organic compound and secondary amide with molecular formula CH3NHCHO, which is a liquid at room temperature.
NMF is mainly used as a reagent in various organic syntheses with limited applications as a highly polar solvent.
The two principal resonance structures for one of these rotamers is shown: This description highlights the partial double bond that exists between the carbonyl carbon and nitrogen, which raises the rotational barrier.
Thus, the molecule is not able to freely rotate around its main axis and the (E)-configuration is preferred due to steric repulsion of the larger substituents.
These reactions can generally be categorized by the following equation: NMF is the precursor to methyl isocyanide, a ligand in coordination chemistry.