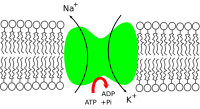
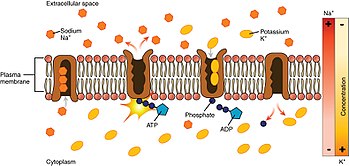
Sodium–potassium pump
[1] The sodium–potassium pump was discovered in 1957 by the Danish scientist Jens Christian Skou, who was awarded a Nobel Prize for his work in 1997.
[3] It also functions as a signal transducer/integrator to regulate the MAPK pathway, reactive oxygen species (ROS), as well as intracellular calcium.
[citation needed] Export of sodium ions from the cell provides the driving force for several secondary active transporters such as membrane transport proteins, which import glucose, amino acids and other nutrients into the cell by use of the sodium ion gradient.
For instance, a study investigated the function of Na+/K+-ATPase in foot muscle and hepatopancreas in land snail Otala lactea by comparing the active and estivating states.
The downstream signals through ouabain-triggered protein phosphorylation events include activation of the mitogen-activated protein kinase (MAPK) signal cascades, mitochondrial reactive oxygen species (ROS) production, as well as activation of phospholipase C (PLC) and inositol triphosphate (IP3) receptor (IP3R) in different intracellular compartments.
For example, the Na+-K+ pump interacts directly with Src, a non-receptor tyrosine kinase, to form a signaling receptor complex.
Based on this scenario, NaKtide, a peptide Src inhibitor derived from the Na+-K+ pump, was developed as a functional ouabain–Na+-K+ pump-mediated signal transduction.
[20] This suggests that the pump might not simply be a homeostatic, "housekeeping" molecule for ionic gradients, but could be a computation element in the cerebellum and the brain.
[21] Indeed, a mutation in the Na+-K+ pump causes rapid onset dystonia-parkinsonism, which has symptoms to indicate that it is a pathology of cerebellar computation.
[22] Furthermore, an ouabain block of Na+-K+ pumps in the cerebellum of a live mouse results in it displaying ataxia and dystonia.
Note: Early studies indicated the opposite effect, but these were later found to be inaccurate due to additional complicating factors.
[citation needed] The Na+/K+-ATPase is endogenously negatively regulated by the inositol pyrophosphate 5-InsP7, an intracellular signaling molecule generated by IP6K1, which relieves an autoinhibitory domain of PI3K p85α to drive endocytosis and degradation.
According to the Blaustein-hypothesis,[31] this carrier enzyme (Na+/Ca2+ exchanger, NCX) uses the Na gradient generated by the Na+-K+ pump to remove Ca2+ from the intracellular space, hence slowing down the Na+-K+ pump results in a permanently elevated Ca2+ level in the muscle, which may be the mechanism of the long-term inotropic effect of cardiac glycosides such as digoxin.
In the case of patients where the heart is not pumping hard enough to provide what is needed for the body, use of digoxin helps to temporarily overcome this.
Na+/K+-ATPase was proposed by Jens Christian Skou in 1957 while working as assistant professor at the Department of Physiology, University of Aarhus, Denmark.
[39] Several studies have detailed the evolution of cardiotonic steroid resistance of the alpha-subunit gene family of Na/K-ATPase (ATP1A) in vertebrates via amino acid substitutions most often located in the first extracellular loop domain.
[40][41][42][43][44][45][46] Amino acid substitutions conferring cardiotonic steroid resistance have evolved independently many times in all major groups of tetrapods.
[47][40][41][42] In Drosophila melanogaster, the alpha-subunit of Na+/K+-ATPase has two paralogs, ATPα (ATPα1) and JYalpha (ATPα2), resulting from an ancient duplication in insects.