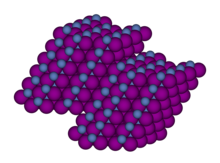
Nickel(II) iodide
This paramagnetic black solid dissolves readily in water to give bluish-green solutions,[1] from which crystallizes the aquo complex [Ni(H2O)6]I2 (image above).
[2] This bluish-green colour is typical of hydrated nickel(II) compounds.
The anhydrous material crystallizes in the CdCl2 motif, featuring octahedral coordination geometry at each Ni(II) center.
The anhydrous form can be produced by treating powdered nickel with iodine.
[5] It is also has niche uses as a reagent in organic synthesis, especially in conjunction with samarium(II) iodide.