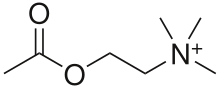
Nicotinic acetylcholine receptor
In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction.
In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways.
Thus, for example, nicotinic receptor antagonists interfere with the baroreflex[9] that normally corrects changes in blood pressure by sympathetic and parasympathetic stimulation of the heart.
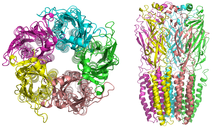
Nicotinic receptors, with a molecular mass of 290 kDa,[10] are made up of five subunits, arranged symmetrically around a central pore.
In the muscle-type receptors, found at the neuromuscular junction, receptors are either the embryonic form, composed of α1, β1, γ, and δ subunits in a 2:1:1:1 ratio ((α1)2β1γδ), or the adult form composed of α1, β1, δ, and ε subunits in a 2:1:1:1 ratio ((α1)2β1δε).
[13] A number of electron microscopy and x-ray crystallography studies have provided very high resolution structural information for muscle and neuronal nAChRs and their binding domains.
[13] In muscle-type nAChRs, the acetylcholine binding sites are located at the α and either ε or δ subunits interface.
[19] Opening of the channel allows positively charged ions to move across it; in particular, sodium enters the cell and potassium exits.
The nAChR is a non-selective cation channel, meaning that several different positively charged ions can cross through.
These α-neurotoxins antagonistically bind tightly and noncovalently to nAChRs of skeletal muscles and in neurons, thereby blocking the action of ACh at the postsynaptic membrane, inhibiting ion flow and leading to paralysis and death.
Progress in discovering the dynamics of binding action of these sites has proved difficult, although recent studies using normal mode dynamics[24] have aided in predicting the nature of both the binding mechanisms of snake toxins and of ACh to nAChRs.
On one hand, the movement of cations causes a depolarization of the plasma membrane (which results in an excitatory postsynaptic potential in neurons) leading to the activation of voltage-gated ion channels.
[30] Desensitized receptors can revert to a prolonged open state when an agonist is bound in the presence of a positive allosteric modulator, for example PNU-120,596.
The neuronal forms of the receptor can be found both post-synaptically (involved in classical neurotransmission) and pre-synaptically[34] where they can influence the release of multiple neurotransmitters.
Neuronal nAChRs are transmembrane proteins that form pentameric structures assembled from a family of subunits composed of α2–α10 and β2–β4.
[40] The pentameric assembly of nAChRs is subjected to the subunits that are produced in various cell types such as in the human lung where epithelial and muscular pentamers largely differ.
Genetic studies have identified single nucleotide polymorphisms (SNPs) in the chromosomal locus encoding these three nAChR genes as risk factors for nicotine dependence, lung cancer, chronic obstructive pulmonary disease, alcoholism, and peripheral arterial disease.
The nAChR subunits encoded by this locus form the predominant nicotinic receptor subtypes expressed in the peripheral nervous system (PNS) and other key central nervous system (CNS) sites, such as the medial habenula, a structure between the limbic forebrain and midbrain involved in major cholinergic circuitry pathways.
[42] Genetic variation in this region also displays influence susceptibility to use drugs of abuse, including cocaine and alcohol consumption.
[44] Nicotinic receptors containing α6 or β3 subunits expressed in brain regions, especially in the ventral tegmental area and substantia nigra, are important for drug behaviors due to their role in dopamine release.
[42][46] Several studies have reported an association between CHRNA7 and endophenotypes of psychiatric disorders and nicotine dependence, contributing to the significant clinical relevance of α7 and research being done on it.