Nitroglycerin
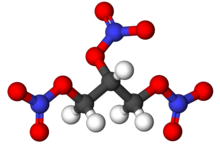
Nitroglycerin (NG) (alternative spelling nitroglycerine), also known as trinitroglycerol (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless or pale yellow, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester.
Discovered in 1846 by Ascanio Sobrero,[4] nitroglycerin has been used as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries.
[5] With a melting point of 12.8 °C, the chemical is almost always encountered as a thick and viscous fluid, changing to a crystalline solid when frozen.
[5][6] Although the pure compound itself is colorless, in practice the presence of nitric oxide impurities left over during production tends to give it a slight yellowish tint.
Due to its high boiling point and consequently low vapor pressure (0.00026 mmHg at 20 °C),[5] pure nitroglycerin has practically no odor at room temperature, with a sweet and burning taste when ingested.
Unintentional detonation may ensue when dropped, shaken, lit on fire, rapidly heated, exposed to sunlight and ozone, subjected to sparks and electrical discharges, or roughly handled.
Though it was previously known that these beneficial effects are due to nitroglycerin being converted to nitric oxide, a potent venodilator, the enzyme for this conversion was only discovered to be mitochondrial aldehyde dehydrogenase (ALDH2) in 2002.
This business exported a liquid combination of nitroglycerin and gunpowder called "Blasting Oil", but this was extremely unstable and difficult to handle, as evidenced in numerous catastrophes.
[14] In April 1866, several crates of nitroglycerin were shipped to California, three of which were destined for the Central Pacific Railroad, which planned to experiment with it as a blasting explosive to expedite the construction of the 1,659-foot-long (506 m) Summit Tunnel through the Sierra Nevada Mountains.
One of the remaining crates exploded, destroying a Wells Fargo company office in San Francisco and killing 15 people.
[15] On Christmas Day 1867, an attempt to dispose of nine canisters of Blasting Oil that had been illegally stored at the White Swan Inn in the centre of Newcastle upon Tyne resulted in an explosion on the Town Moor that killed eight people.
In June 1869, two one-ton wagons loaded with nitroglycerin, then known locally as Powder-Oil, exploded in the road at the North Wales village of Cwm-y-glo.
The UK Government was so alarmed at the damage caused and what could have happened in a city location (these two tons were part of a larger load coming from Germany via Liverpool) that they soon passed the Nitro-Glycerine Act of 1869.
[16] Liquid nitroglycerin was widely banned elsewhere, as well, and these legal restrictions led to Alfred Nobel and his company's developing dynamite in 1867.
Similar mixtures, such as "dualine" (1867), "lithofracteur" (1869), and "gelignite" (1875), were formed by mixing nitroglycerin with other inert absorbents, and many combinations were tried by other companies in attempts to get around Nobel's tightly held patents for dynamite.
He began treating his patients with small diluted doses of nitroglycerin in 1878, and this treatment was soon adopted into widespread use after Murrell published his results in the journal The Lancet in 1879.
[23] Ethylene glycol dinitrate or another polynitrate may be added to lower the melting point and thereby avoid the necessity of thawing frozen explosive.
[24] Chemically "desensitizing" nitroglycerin is possible to a point where it can be considered about as "safe" as modern high explosives, such as by the addition of ethanol, acetone, or dinitrotoluene.
The nitrator vessel, often constructed of iron or lead and generally stirred with compressed air, has an emergency trap door at its base, which hangs over a large pool of very cold water and into which the whole reaction mixture (called the charge) can be dumped to prevent an explosion, a process referred to as drowning.
If the temperature of the charge exceeds about 30 °C (86 °F) (actual value varying by country) or brown fumes are seen in the nitrator's vent, then it is immediately drowned.
Dynamite and similar explosives were widely adopted for civil engineering tasks, such as in drilling highway and railroad tunnels, for mining, for clearing farmland of stumps, in quarrying, and in demolition work.
Triple-base propellants contain nitrocellulose, nitroglycerin, and nitroguanidine, but are reserved mainly for extremely high-caliber ammunition rounds such as those used in tank cannons and naval artillery.
Blasting gelatin, also known as gelignite, was invented by Nobel in 1875, using nitroglycerin, wood pulp, and sodium or potassium nitrate.
At low doses, nitroglycerin dilates veins more than arteries, thereby reducing preload (volume of blood in the heart after filling); this is thought to be its primary mechanism of action.
[35] An improved ratio of myocardial oxygen demand to supply leads to the following therapeutic effects during episodes of angina pectoris: subsiding of chest pain, decrease of blood pressure, increase of heart rate, and orthostatic hypotension.
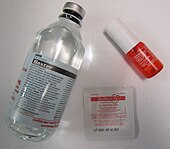
[36][37] Nitroglycerin is available in tablets, ointment, solution for intravenous use, transdermal patches, or sprays administered sublingually.
These headaches can be severe enough to incapacitate some people; however, humans develop a tolerance to and dependence on nitroglycerin after long-term exposure.
Over the weekend, the workers lose the tolerance, and when they are re-exposed on Monday, the drastic vasodilation produces a fast heart rate, dizziness, and a headache.