Chemical polarity
Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points.
To determine the polarity of a covalent bond using numerical means, the difference between the electronegativity of the atoms is used.
[3] As a quantum-mechanical description, Pauling proposed that the wave function for a polar molecule AB is a linear combination of wave functions for covalent and ionic molecules: ψ = aψ(A:B) + bψ(A+B−).
The amount of covalent and ionic character depends on the values of the squared coefficients a2 and b2.
Based on the conversion factor of 10−10 statcoulomb being 0.208 units of elementary charge, so 1.0 debye results from an electron and a proton separated by 0.208 Å.
A molecule may be polar either as a result of polar bonds due to differences in electronegativity as described above, or as a result of an asymmetric arrangement of nonpolar covalent bonds and non-bonding pairs of electrons known as a full molecular orbital.
[citation needed] Polar liquids have a tendency to rise against gravity in a small diameter tube.
[citation needed] For example, nonpolar hexane is much less viscous than polar water.
In the gas phase the dipole moment is ≈ 1.86 debye (D),[11] whereas liquid water (≈ 2.95 D)[12] and ice (≈ 3.09 D)[13] are higher due to differing hydrogen-bonded environments.
For example, the water molecule (H2O) contains two polar O−H bonds in a bent (nonlinear) geometry.
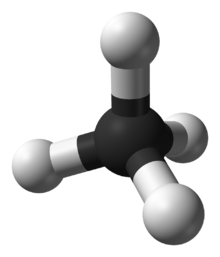
The molecule has two lone electrons in an orbital that points towards the fourth apex of an approximately regular tetrahedron, as predicted by the VSEPR theory.
This orbital is not participating in covalent bonding; it is electron-rich, which results in a powerful dipole across the whole ammonia molecule.
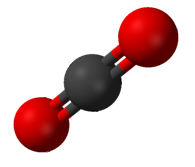
In ozone (O3) molecules, the two O−O bonds are nonpolar (there is no electronegativity difference between atoms of the same element).
For example, boron trifluoride (BF3) has a trigonal planar arrangement of three polar bonds at 120°.
They are good surfactants and can aid in the formation of stable emulsions, or blends, of water and fats.
Surfactants reduce the interfacial tension between oil and water by adsorbing at the liquid–liquid interface.
Any molecule with a centre of inversion ("i") or a horizontal mirror plane ("σh") will not possess dipole moments.
As a consequence of that constraint, all molecules with dihedral symmetry (Dn) will not have a dipole moment because, by definition, D point groups have two or multiple Cn axes.
Contrary to popular misconception, the electrical deflection of a stream of water from a charged object is not based on polarity.
A stream of water can also be deflected in a uniform electrical field, which cannot exert force on polar molecules.