Non-covalent interaction
The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/mol (1000–5000 calories per 6.02×1023 molecules).
[2] Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.
[3][2] Non-covalent interactions[4] are critical in maintaining the three-dimensional structure of large molecules, such as proteins and nucleic acids.
They are also involved in many biological processes in which large molecules bind specifically but transiently to one another (see the properties section of the DNA page).
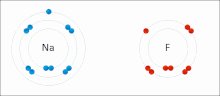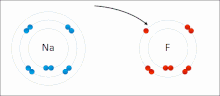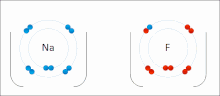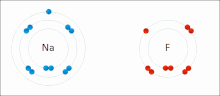
Ionic interactions involve the attraction of ions or molecules with full permanent charges of opposite signs.
Most commonly, the strength of hydrogen bonds lies between 0–4 kcal/mol, but can sometimes be as strong as 40 kcal/mol[3] In solvents such as chloroform or carbon tetrachloride one observes e.g. for the interaction between amides additive values of about 5 kJ/mol.
Measurements of thousands of complexes in chloroform or carbon tetrachloride have led to additive free energy increments for all kind of donor-acceptor combinations.
The nucleophilic agent in these interactions tends to be highly electronegative (such as oxygen, nitrogen, or sulfur), or may be anionic, bearing a negative formal charge.
[5] Van der Waals forces are a subset of electrostatic interactions involving permanent or induced dipoles (or multipoles).
For example, acetone, the active ingredient in some nail polish removers, has a net dipole associated with the carbonyl (see figure 2).
[citation needed] London dispersion forces[14][15][16][17] are the weakest type of non-covalent interaction.
[6] Hexane is a good example of a molecule with no polarity or highly electronegative atoms, yet is a liquid at room temperature due mainly to London dispersion forces.
[3] The high polarizability of aromatic rings lead to dispersive interactions as major contribution to so-called stacking effects.
[3] The sandwich configuration is not nearly as stable of an interaction as the previously two mentioned due to high electrostatic repulsion of the electrons in the π orbitals.
In this case, an anion sits atop an electron-poor π-system, usually established by the presence of electron-withdrawing substituents on the conjugated molecule[21] Polar–π interactions involve molecules with permanent dipoles (such as water) interacting with the quadrupole moment of a π-system (such as that in benzene (see figure 5).
[citation needed] The hydrophobic effect is the desire for non-polar molecules to aggregate in aqueous solutions in order to separate from water.
The binding of a small molecule to a protein is governed by a combination of steric, or spatial considerations, in addition to various non-covalent interactions, although some drugs do covalently modify an active site (see irreversible inhibitors).
For example, dinuclear triple-helical compounds in which three ligand strands wrap around two metals, resulting in a roughly cylindrical tetracation have been prepared.
[29] The folding of proteins from a primary (linear) sequence of amino acids to a three-dimensional structure is directed by all types of non-covalent interactions, including the hydrophobic forces and formation of intramolecular hydrogen bonds.
For example, consider three compounds of similar chemical composition: sodium n-butoxide (C4H9ONa), diethyl ether (C4H10O), and n-butanol (C4H9OH).