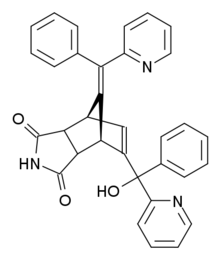
Norbormide
It has several mechanisms of action, acting as a vasoconstrictor and calcium channel blocker,[1] but is selectively toxic to rats and has relatively low toxicity to other species, due to a species specific action of opening the permeability transition pores in rat mitochondria.
During the 1970s, however, the utilization of this rodenticide decreased, since anticoagulant toxins seemed to be more effective against a wider range of rodents.
[5] Although many view its selective feature as a disadvantage, scientists of Landcare Research in New Zealand search for ways to improve this rodenticide and develop several analogues.
The imide ring can have an endo or an exo orientation and for the hydroxyl group erythro and threo isomers are possible.
Only substitution of the NH proton of the imide with certain groups could give toxic activity comparable to norbormide itself.
Probably the influx of calcium is mediated by the phospholipase C (PLC)-coupled receptors, in rat peripheral artery myocytes.
[13][14] Other research of Sergio Bova et al. has shown that in respiratory, urinary and gastrointestinal smooth muscle there was no contraction by norbormide.
This PTP is an inner mitochondrial membrane (IMM) channel, whose opening leads to an increasing permeability for ions with an exclusion size of about 1500 Da.
[17][18][19] In vitro studies on liver preparations from rats and other rodents revealed that hydroxylation is the major process during the metabolism of NRB.
[22] A mixture of four active endo-isomers of NRB (U, V, W en Y) formed four major metabolites in rat liver S9 and cytosolic preparations after incubation.
[5] The same metabolites were found in guinea pigs, although the levels of metabolism in these rodents are considerately lower compared to those in rats.
[23] After NRB had orally been administered, an examination of both rat and mouse liver revealed traces of the parent compound.
This lack of detectable metabolites implies that NRB is able to induce a lethal effect at extremely low levels.
[24] Another study has shown that there were respiratory depressions after the cardiovascular effects in the rats which were treated with norbormide in vivo.
In all animals tested and also in the rat aorta and extravascular smooth muscle tissue, NRB exhibits vasorelaxant properties in the arteries.
[13] Another effect of NRB is stimulation of corticosterone and aldosterone production in both rat and mice adrenal gland by enhancing late steps of steroid-hormone synthesis.
[14] The unique toxicity of NRB has been determined by performing animal experiments by using several species of rodents.
Their breathing begins to be laboured and within a short period of time the rats suffer from series of convulsive movements.
The spasm is followed by death, which occurs within 30 minutes in albino laboratory rats and with two hours in wild animals.
These irreversible effects weren't marked in the heart muscle (myocardium) as perhaps expected, but straight in the coronary arteries.
[25] Recent studies present NRB's ability to activate the mitochondrial permeability transition pore (MPTP) in isolated rat preparations.
It is understandable that such an abnormity in the mitochondrial membranes bring about issues in the cellular metabolism of the poisoned animal.
[8] The members of the genus Rattus are highly sensitive to NRB, but other animals experience no toxic effects.
Upon closer examination a 1000 mg/kg dose of norbormide didn't bring forth any toxic signs when orally administered to cats, chickens, dogs, monkeys, mice, swine or birds.