Nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile).
[1][2] The most general form of the reaction may be given as the following: The electron pair (:) from the nucleophile (Nuc) attacks the substrate (R−LG) and bonds with it.
[3] In 1935, Edward D. Hughes and Sir Christopher Ingold studied nucleophilic substitution reactions of alkyl halides and related compounds.
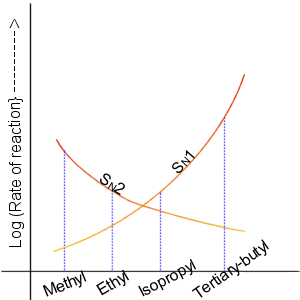
SN2 occurs when the central carbon atom is easily accessible to the nucleophile.
First of all, the 2 in SN2 implies that there are two concentrations of substances that affect the rate of reaction: substrate (Sub) and nucleophile.
SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation.
Common examples include: An example of a substitution reaction taking place by a so-called borderline mechanism as originally studied by Hughes and Ingold[6] is the reaction of 1-phenylethyl chloride with sodium methoxide in methanol.
The SNi mechanism is observed in reactions of thionyl chloride with alcohols, and it is similar to SN1 except that the nucleophile is delivered from the same side as the leaving group.
This may be seen in the reaction of 1-chloro-2-butene with sodium hydroxide to give a mixture of 2-buten-1-ol and 1-buten-3-ol: The Sn1CB mechanism appears in inorganic chemistry.
Nucleophilic substitution via the SN1 or SN2 mechanism does not generally occur with vinyl or aryl halides or related compounds.