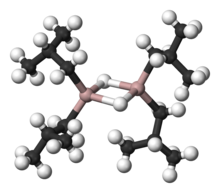
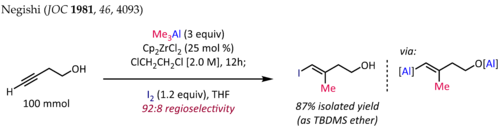Organoaluminium chemistry
The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species.
Organoaluminium compounds generally feature three- and four-coordinate Al centers, although higher coordination numbers are observed with inorganic ligands such as fluoride.
[5] The high Lewis acidity of the monomeric species is related to the size of the Al(III) center and its tendency to achieve an octet configuration.
They are typically prepared reduction of the dialkylaluminium chlorides by metallic potassium:[6] Another notable group of alanes are tetraalanes containing four Al(I) centres.
[7] The overall reaction for the production of these simple alkylaluminium compounds is thus as follows: Aluminium powder reacts directly with certain terminal alkenes in the presence of hydrogen.
[8] The so-called ZACA reaction first reported by Ei-ichi Negishi is an example of an asymmetric carboalumination of alkenes catalyzed by a chiral zirconocene catalyst.
[9] The methylalumination of alkynes in the presence of Cp2ZrCl2[10][11] is employed for the synthesis of stereodefined trisubstituted olefin fragments, a common substructure in terpene and polyketide natural products.
Although the simple members are commercially available at low cost, many methods have been developed for their synthesis in the laboratory, including metathesis or transmetalation.
The high reactivity of organoaluminium compounds toward electrophiles is attributed to the charge separation between aluminium and carbon atom.
Organoaluminium compounds are hard acids and readily form adducts with bases such as pyridine, THF and tertiary amines.
[16] With oxygen one obtains the corresponding alkoxides, which can be hydrolysed to the alcohols: A structurally characterized organoaluminum peroxide is [{HC[C(Me)N-C6H5]2}Al(R)-O-O-CMe3] [R=CH(SiMe3)2].