Organobismuth chemistry
[6] The chemistry of these complexes first began receiving significant attention when Grignard reagents and organolithium compounds became available.
For instance, Barton and coworkers demonstrated that amines could be N-arylated with a bismuth(III) reagent in the presence of copper(II) salt.
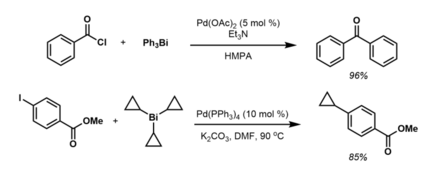
[15] Although not formally arenes, tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes:[16] The thermal stability of R5M compounds decrease in the order As > Sb > Bi.
[citation needed] The nature of the aryl ligands is important in determining whether the complex's geometry is trigonal bipyramidal or square planar and its color.
[6] All-carbon organobismuth(V) complexes may then be accessed from displacement of the newly formed bismuth-halogen bond with an alkyl or aryl lithium or Grignard reagent.
Compared to their lighter congeners, Bi(V) compounds are strong oxidants, dehydrogenating alcohols of all kinds to the carbonyl and cleaving glycols to the aldehyde.
[21][page needed] In general, oxidation accelerates when the aryl ligands have electron-withdrawing substituents, and attacks alcohols before thiols or selenides.
[8]: 417–418 High yields require strongly basic conditions (absent it, the carbonate is the most effective), suggesting that the active species are triarylbismuth oxides.
[8]: 416–417 In general, bismuth(V) compounds arylate inefficiently, transferring only one of the five ligands to the substrate[22] and leaving behind a triarylbismuth(III) waste.
[23] Reoxidizing the BiIII complex to BiV is hard, and impedes closing a catalytic cycle around this chemistry.
The subsequent arylation and elimination are asynchronous and concerted:[23] Adjacent electron-pair donors determine regioselectivity.
[8]: 417–431 Bismuthinidene are analogous to carbenes since they have the general form R-Bi, with two lone pairs of electrons on the central bismuth(I) atom.