Organoiridium chemistry
[2] Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid.
They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.
[3] Iridium(0) complexes are binary carbonyls, the principal member being tetrairidium dodecacarbonyl, Ir4(CO)12.
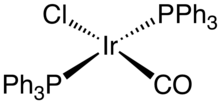
Other common complexes include Ir2Cl2(cyclooctadiene)2, chlorobis(cyclooctene)iridium dimer, The analogue of Wilkinson's catalyst, IrCl(PPh3)3), undergoes orthometalation: This difference between RhCl(PPh3)3 and IrCl(PPh3)3 reflects the generally greater tendency of iridium to undergo oxidative addition.
Important starting reagents being hydrated iridium trichloride and ammonium hexachloroiridate.
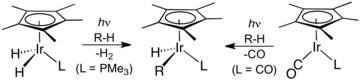
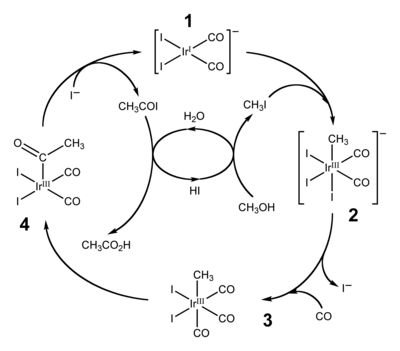
Illustrative is the carbonylation of the trichloride: IrCl3(H2O)x + 3 CO → [Ir(CO)2Cl2]− + CO2 + 2 H+ + Cl− + (x-1) H2O Many organoiridium(III) compounds are generated from pentamethylcyclopentadienyl iridium dichloride dimer.
[12] The dominant application of organoiridium complexes is as catalyst in the Cativa process for carbonylation of methanol to produce acetic acid.