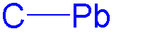Organolead chemistry
[2] By far the organolead compound that has had the greatest impact is tetraethyllead, formerly used as an antiknock agent in gasoline intended for automobile internal combustion engines and still widely used in avgas for small aircraft.
Derived from the hydroxides are the plumboxanes: which give access to polymeric alkoxides: The C–Pb bond is weak and for this reason homolytic cleavage of organolead compounds to free radicals is easy.
General reaction types of aryl and vinyl organoleads are transmetalation for instance with boronic acids and acid-catalyzed heterocyclic cleavage.
The lead substituent in p-methoxyphenyllead triacetate is displaced by carbon nucleophiles, such as the phenol mesitol, exclusively at the aromatic ortho position:[6] The reaction requires the presence of a large excess of a coordinating amine such as pyridine which presumably binds to lead in the course of the reaction.
The second step is then akin to a Claisen rearrangement except that the reaction depends on the electrophilicity (hence the ortho preference) of the phenol.
The second step is reductive elimination with formation of a new C–C bond and lead(II) acetate.