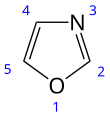
Oxazole
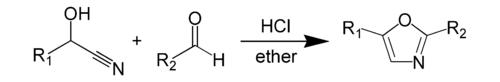
The classic synthetic route the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones: The Fischer oxazole synthesis from cyanohydrins and aldehydes is also widely used: Other methods are known including the reaction of α-haloketones and formamide and the Van Leusen reaction with aldehydes and TosMIC.
Deprotonation of oxazoles occurs at C2, and the lithio salt exists in equilibrium with the ring-opened enolate-isonitrile, which can be trapped by silylation.
Electrophilic aromatic substitution takes place at C5, but requiring electron donating groups.
Diels–Alder reactions involving oxazole (as dienes) and electrophilic alkenes has been well developed as a route to pyridines.
The initial cycloaddition affords a bicyclic intermediate, with an acid-sensitive oxo bridgehead.





3 for serine and threonine respectively, B = base.
(1) Enzymatic cyclization. (2) Elimination. (3) [O] = enzymatic oxidation.
