Oxidative coupling of phenols
Oxidative phenol couplings are often catalyzed by transition metal complexes including V, Cr, Mn, Cu, Fe, among others.
The resulting reactive intermediate can engage in downstream chemical processes which can occur via either coordinated (inner-sphere) or non-coordinated coupling partners.
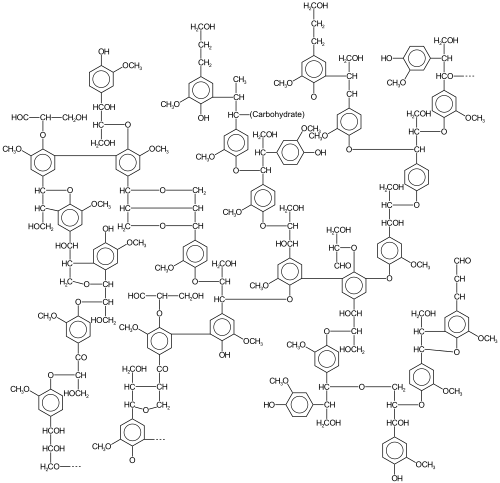
Lignin, a polyphenol that is found in most plants, is a very abundant form of biomass that arises, in part, by oxidative coupling of phenols.
Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily.
The reaction is attractive for their atom economy because it avoid pre-functionalized starting materials often required in traditional redox-neutral cross-couplings.
[9] Moreover, stereoselectivity is an important consideration if the resulting biphenol compound displays axial chirality or atropoisomerism.
Oxidative couplings have also been studied between phenols and nonphenolic compounds including anilines, beta-ketoesters/malonates/malononitriles, electron-rich arenes, olefins, and other functional groups.