Lipid
Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups.
Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, monoglycerides, and phospholipids), as well as other sterol-containing metabolites such as cholesterol.
In 1815, Henri Braconnot classified lipids (graisses) in two categories, suifs (solid greases or tallow) and huiles (fluid oils).
[11] Several years later, Marcellin Berthelot, one of Pelouze's students, synthesized tristearin and tripalmitin by reaction of the analogous fatty acids with glycerin in the presence of gaseous hydrogen chloride at high temperature.
[12] In 1827, William Prout recognized fat ("oily" alimentary matters), along with protein ("albuminous") and carbohydrate ("saccharine"), as an important nutrient for humans and animals.
[13][14] For a century, chemists regarded "fats" as only simple lipids made of fatty acids and glycerol (glycerides), but new forms were described later.
[17][18] The word lipide, which stems etymologically from Greek λίπος, lipos 'fat', was introduced in 1923 by the French pharmacologist Gabriel Bertrand.
[9] The word lipide was unanimously approved by the international commission of the Société de Chimie Biologique during the plenary session on July 3, 1923.
If a fatty acid contains a double bond, there is the possibility of either a cis or trans geometric isomerism, which significantly affects the molecule's configuration.
Three double bonds in 18-carbon linolenic acid, the most abundant fatty-acyl chains of plant thylakoid membranes, render these membranes highly fluid despite environmental low-temperatures,[24] and also makes linolenic acid give dominating sharp peaks in high resolution 13-C NMR spectra of chloroplasts.
The hydrolysis of the ester bonds of triglycerides and the release of glycerol and fatty acids from adipose tissue are the initial steps in metabolizing fat.
[31]: 630–1 Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage.
[35] Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders.
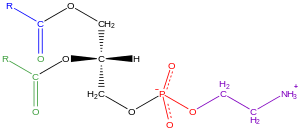
[37] Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer).
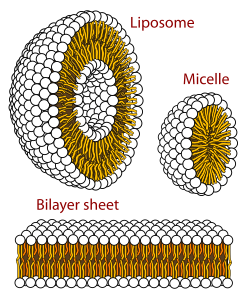
In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of or, themselves, membrane-derived second messengers.
[31]: 844 Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.
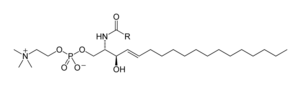
[42] The glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base.
[49] Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin.
[51] Saccharolipids describe compounds in which fatty acids are linked to a sugar backbone, forming structures that are compatible with membrane bilayers.
The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.
They comprise many secondary metabolites and natural products from animal, plant, bacterial, fungal and marine sources, and have great structural diversity.
[58] Plant thylakoid membranes have the largest lipid component of a non-bilayer forming monogalactosyl diglyceride (MGDG), and little phospholipids; despite this unique lipid composition, chloroplast thylakoid membranes have been shown to contain a dynamic lipid-bilayer matrix as revealed by magnetic resonance and electron microscope studies.
[70] These include sphingosine-1-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization,[71] cell growth, and apoptosis;[72] diacylglycerol and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C;[73] the prostaglandins, which are one type of fatty-acid derived eicosanoid involved in inflammation and immunity;[74] the steroid hormones such as estrogen, testosterone and cortisol, which modulate a host of functions such as reproduction, metabolism and blood pressure; and the oxysterols such as 25-hydroxy-cholesterol that are liver X receptor agonists.
They accomplish this by being exposed to the extracellular face of the cell membrane after the inactivation of flippases which place them exclusively on the cytosolic side and the activation of scramblases, which scramble the orientation of the phospholipids.
[76] The "fat-soluble" vitamins (A, D, E and K) – which are isoprene-based lipids – are essential nutrients stored in the liver and fatty tissues, with a diverse range of functions.
The acetyl-CoA is then ultimately converted into adenosine triphosphate (ATP), CO2, and H2O using the citric acid cycle and the electron transport chain.
Alpha-linolenic acid is found in the green leaves of plants and in some seeds, nuts, and legumes (in particular flax, rapeseed, walnut, and soy).
[91]: 388 Many studies have shown positive health benefits associated with consumption of omega-3 fatty acids on infant development, cancer, cardiovascular diseases, and various mental illnesses (such as depression, attention-deficit hyperactivity disorder, and dementia).
[93][94] In contrast, it is now well-established that consumption of trans fats, such as those present in partially hydrogenated vegetable oils, are a risk factor for cardiovascular disease.
[101][102] None of these studies suggested any connection between percentage of calories from fat and risk of cancer, heart disease, or weight gain.