Oxidosqualene cyclase
Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.
[1] There are two major groups of sterol-producing OSC enzymes: Sterols and triterpenes are extremely diverse classes of natural products, particularly in plants, which often contain numerous OSC enzymes with different substrate and product specificities;[1] common examples include lupeol synthase and beta-amyrin synthase.
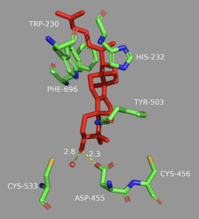
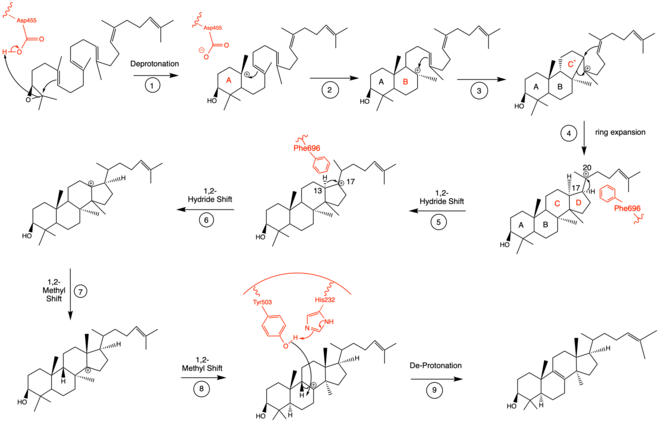
[6] The residues in the active site make it energetically favorable for oxidosqualene to take on a more folded conformation, which closely resembles its product.
[15] To note, QM/MM studies have shown that the protonation of this epoxide ring is the rate limiting step of the entire mechanism,[13] indicating it may be the most regulated.
[16] Molecular dynamics simulations have proved critical to understanding the conformational changes that oxidosqualene-lanosterol intermediates undergo.
The literature has shown that two amino acids in OSC are critical for this de-protonation to occur correctly and uniformly.
[13] High blood cholesterol, also called hypercholesterolemia, significantly increases the risk of stroke, heart attack, and peripheral artery disease.
Research is being done for other compounds which block different steps in the biosynthesis of cholesterol, including the reaction performed by oxidosqualene cyclase which cyclizes squalene to form lanosterol.
[20] Another parasite, Leishmania donovani, is the causative agent for leishmaniasis, a similar tropical disease that is spread by sand flies and disproportionately affects rural areas.
[21] GSK2920487A, a cyclic and aromatic small molecule, has been shown to significantly inhibit oxidosqualene cyclase and decrease the survival of the L.
[22] While there is concern that parasites such as T. cruzi and L. donovani are able to scavenge the sterol compounds that oxidosqualene cyclase catalyzes the formation of, the potency of chemical inhibition against this vital enzyme demonstrate the potential of OSC as a therapeutic target.
However, this enzyme is necessary for OSC function in yeast only, suggesting the divergent evolution of the steroid biosynthesis pathway in mammals.