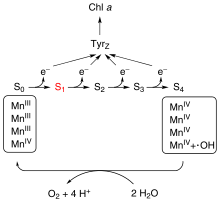
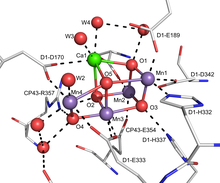
Oxygen-evolving complex
[4] Based on a widely accepted theory from 1970 by Kok, the complex can exist in 5 states, denoted S0 to S4, with S0 the most reduced and S4 the most oxidized.
S4 reacts with water producing free oxygen: This conversion resets the catalyst to the S0 state.
The active site of the OEC consists of a cluster of manganese and calcium with the formula Mn4Ca1OxCl1–2(HCO3)y.
Many characteristics of it have been examined by flash photolysis experiments, electron paramagnetic resonance (EPR), and X-ray spectroscopy.
[5] The mechanism of the complex is proposed to involve an Mn-oxide which couples by O-O bond formation to a calcium oxide/hydroxide.