Peripheral membrane protein
The reversible attachment of proteins to biological membranes has shown to regulate cell signaling and many other important cellular events, through a variety of mechanisms.
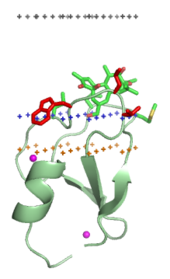
The inner and outer surfaces, or interfacial regions, of model phospholipid bilayers have been shown to have a thickness of around 8 to 10 Å, although this may be wider in biological membranes that include large amounts of gangliosides or lipopolysaccharides.
[7] The hydrophobic inner core region of typical biological membranes may have a thickness of around 27 to 32 Å, as estimated by Small angle X-ray scattering (SAXS).
[11] Some water-soluble proteins associate with lipid bilayers irreversibly and can form transmembrane alpha-helical or beta-barrel channels.
It also may involve the formation or dissociation of protein quaternary structures or oligomeric complexes, and specific binding of ions, ligands, or regulatory lipids.
Typical amphitropic proteins must interact strongly with the lipid bilayer in order to perform their biological functions.
These proteins may be anchored to the bilayer as a result of hydrophobic interactions between the bilayer and exposed nonpolar residues at the surface of a protein,[13] by specific non-covalent binding interactions with regulatory lipids , or through their attachment to covalently bound lipid anchors.
[15] Lipid anchored proteins are covalently attached to different fatty acid acyl chains on the cytoplasmic side of the cell membrane via palmitoylation, myristoylation, or prenylation.
Such complexes are also stabilized by the formation of ionic bridges between the aspartate or glutamate residues of the protein and lipid phosphates via intervening calcium ions (Ca2+).
Such ionic bridges can occur and are stable when ions (such as Ca2+) are already bound to a protein in solution, prior to lipid binding.
These interactions are relatively weak at the physiological ionic strength (0.14M NaCl): ~3 to 4 kcal/mol for small cationic proteins, such as cytochrome c, charybdotoxin or hisactophilin.
However, typical amphitropic proteins have various hydrophobic anchors that penetrate the interfacial region and reach the hydrocarbon interior of the membrane.
Their binding to membranes can be mediated by calcium ions (Ca2+) that form bridges between the acidic protein residues and phosphate groups of lipids, as in annexins or GLA domains.
The transported substances are phosphatidylinositol, tocopherol, gangliosides, glycolipids, sterol derivatives, retinol, fatty acids, water, macromolecules, red blood cells, phospholipids, and nucleotides.
In other cases, the experimental structure represents a water-soluble conformation that interacts with the lipid bilayer peripherally, although some of the channel-forming peptides are rather hydrophobic and therefore were studied by NMR spectroscopy in organic solvents or in the presence of micelles.