Pacemaker
[5] Others, called biventricular pacemakers, have multiple electrodes stimulating different positions within the ventricles (the lower heart chambers) to improve their synchronization.
Thus, the timing between the atrial and ventricular contractions, as well as between the septal and lateral walls of the left ventricle can be adjusted to achieve optimal cardiac function.
CRT devices have been shown to reduce mortality and improve quality of life in patients with heart failure symptoms; a LV ejection fraction less than or equal to 35% and QRS duration on EKG of 120 ms or greater.
[20] Conventional placement of ventricular leads in or around the tip or apex of the right ventricle, or RV apical pacing, can have negative effects on heart function.
By stimulating the His–Purkinje fiber network directly with a special lead and placement technique, HBP causes a synchronized and therefore more effective ventricular activation and avoids long-term heart muscle disease.
[21][22] A major step forward in pacemaker function has been to attempt to mimic nature by utilizing various inputs to produce a rate-responsive pacemaker using parameters such as the QT interval, pO2 – pCO2 (dissolved oxygen or carbon dioxide levels) in the arterial-venous system, physical activity as determined by an accelerometer, body temperature, ATP levels, adrenaline, etc.
The DAVID trials[24] have shown that unnecessary pacing of the right ventricle can exacerbate heart failure and increases the incidence of atrial fibrillation.
However, a 2013 study found that "The overall risk of clinically significant adverse events related to EMI (electromagnetic interference) in recipients of CIEDs (cardiovascular implantable electronic devices) is very low.
A 2008 US study found[35] that the magnetic field created by some headphones used with portable music players or cellphones may cause interference if placed very close to some pacemakers.
[39][40] Some patients consider that hopeless, debilitating conditions, such as severe strokes or late-stage dementia, can cause so much suffering that they would prefer not to prolong their lives with supportive measures.
The proof of concept exploit helps demonstrate the need for better security and patient alerting measures in remotely accessible medical implants.
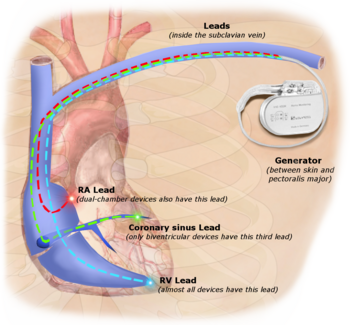
The leads are small-diameter wires from the pacemaker to the implantation site in the heart muscle, and are usually placed intravenously through the subclavian vein in order to access the right atrium.
The lead removal technique will vary depending on the surgeon's estimation of the probability that simple traction will suffice to more complex procedures.
The free end of a pacemaker lead is actually implanted into the heart muscle with a miniature screw or anchored with small plastic hooks called tines.
Overall life expectancy with pacemakers is excellent, and mostly depends upon underlying diseases, presence of atrial fibrillation, age and sex at the time of first implantation.
[58] In 1926, Mark C Lidwill of the Royal Prince Alfred Hospital of Sydney, supported by physicist Edgar H. Booth of the University of Sydney, devised a portable apparatus which "plugged into a lighting point" and in which "One pole was applied to a skin pad soaked in strong salt solution" while the other pole "consisted of a needle insulated except at its point, and was plunged into the appropriate cardiac chamber".
[59] In 1928, the apparatus was used to revive a stillborn infant at Crown Street Women's Hospital in Sydney, whose heart continued "to beat on its own accord", "at the end of 10 minutes" of stimulation.
[64] For example, "Hyman did not publish data on the use of his pacemaker in humans because of adverse publicity, both among his fellow physicians, and due to newspaper reporting at the time.
[67] A number of innovators, including Paul Zoll, made smaller but still bulky transcutaneous pacing devices from 1952 using a large rechargeable battery as the power supply.
In the UK in the 1960s, Lucas Engineering in Birmingham was asked by Mr Abrams of The Queen Elizabeth Hospital to produce a prototype for a transistorised replacement for the electro-mechanical product.
[77] In 1959, temporary transvenous pacing was first demonstrated by Seymour Furman and John Schwedel, whereby the catheter electrode was inserted via the patient's basilic vein.
[78] In February 1960, an improved version of the Swedish Elmqvist design was implanted by Doctors Orestes Fiandra and Roberto Rubio in the Casmu 1 Hospital of Montevideo, Uruguay.
The first use of transvenous pacing in conjunction with an implanted pacemaker was by Parsonnet in the United States,[79][80][81] Lagergren in Sweden[82][83] and Jean-Jacques Welti in France[84] in 1962–63.
The transvenous, or pervenous, procedure involved incision of a vein into which was inserted the catheter electrode lead under fluoroscopic guidance, until it was lodged within the trabeculae of the right ventricle.
Cardiothoracic surgeon Leon Abrams and medical engineer Ray Lightwood developed and implanted the first patient-controlled variable-rate heart pacemaker in 1960 at the University of Birmingham.
[85][86] The preceding implantable devices all suffered from the unreliability and short lifetime of the available primary cell technology, mainly the mercury battery.
A further impediment to the reliability of the early devices was the diffusion of water vapor from body fluids through the epoxy resin encapsulation, affecting the electronic circuitry.
[92] In November 2014, Bill Pike of Fairbanks, Alaska, received a Medtronic Micra pacemaker in Providence St Vincent Hospital in Portland, Oregon.
[95] While the St Jude Nanostim and the Medtronic Micra are single-chamber pacemakers, it was anticipated that leadless dual-chamber pacing for patients with atrioventricular block would become possible with further development.
Pacemakers with significant remaining battery life are potentially life-saving devices for people in low- and middle-income countries (LMICs).