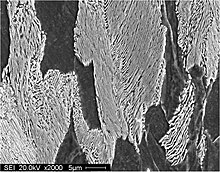
Pearlite
The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the lever rule.
It has been recently shown that cold wire drawing not only strengthens pearlite by refining the lamellae structure, but also simultaneously causes partial chemical decomposition of cementite, associated with an increased carbon content of the ferrite phase, deformation induced lattice defects in ferrite lamellae,[3] and even a structural transition from crystalline to amorphous cementite.
The deformation-induced decomposition and microstructural change of cementite is closely related to several other phenomena such as a strong redistribution of carbon and other alloy elements like silicon and manganese in both the cementite and the ferrite phase; a variation of the deformation accommodation at the phase interfaces due to a change in the carbon concentration gradient at the interfaces; and mechanical alloying.
The carbon diffusion during the formation of pearlite, just ahead of the growth front, is critical in determining the thickness of the lamellae and, consequently, the strength of the steel.
[5] Bainite is a similar structure with lamellae much smaller than the wavelength of visible light and thus lacks this pearlescent appearance.