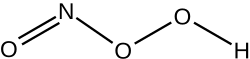
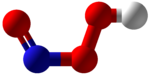
Peroxynitrous acid
Peroxynitrous acid (HNO3) is a reactive nitrogen species (RNS).
It is formed in vivo from the diffusion-controlled reaction of nitrogen monoxide (ON•) and superoxide (O•−2).
It is an isomer of nitric acid and isomerises with a rate constant of k = 1.2 s−1, a process whereby up to 5% of hydroxyl and nitrogen dioxide radicals may be formed.
It oxidises and nitrates aromatic compounds in low yield.
[3] Peroxynitrous acid is also important in atmospheric chemistry.