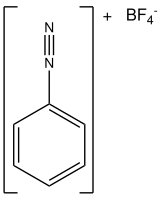
Benzenediazonium tetrafluoroborate
It exists as a colourless solid that is soluble in polar solvents.
It is the parent member of the aryldiazonium compounds,[1] which are widely used in organic chemistry.
A wide range of groups that can be used to replace N2 including halide, SH−, CO2H−, OH−.
Of considerable practical value in the dye industry are the diazo coupling reactions.
[4] Whereas the chloride salt is explosive,[5] the tetrafluoroborate is readily isolated.