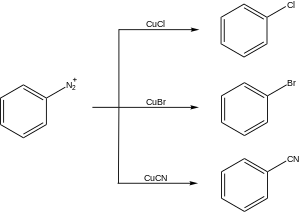
Sandmeyer reaction
The reaction was discovered in 1884 by Swiss chemist Traugott Sandmeyer, when he attempted to synthesize phenylacetylene from benzenediazonium chloride and copper(I) acetylide.
Diazonium salts also react with boronates, iodide, thiols, water, hypophosphorous acid and others,[6] and fluorination can be carried out using tetrafluoroborate anions (Balz–Schiemann reaction).
[7] Due to its wide synthetic applicability, the Sandmeyer reaction, along with other transformations of diazonium compounds, is complementary to electrophilic aromatic substitution.
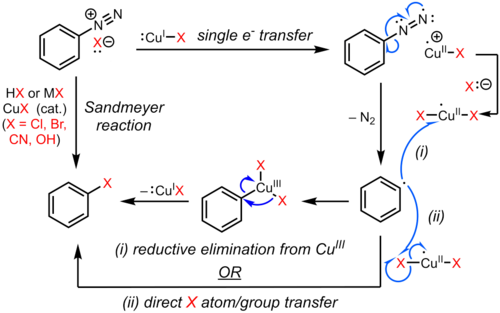
[8] The substitution of the aromatic diazo group with a halogen or pseudohalogen is initiated by a one-electron transfer mechanism catalyzed by copper(I) to form an aryl radical with loss of nitrogen gas.
[12][13][14] However, evidence for such an organocopper intermediate is weak and mostly circumstantial,[15][16] and the exact pathway may depend on the substrate and reaction conditions.
[23] The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target topoisomerase II as an anti-cancer drug.
[28] This is in contrast to the classical procedure (known by the German name Verkochung [de]), which calls for boiling the diazonium salt in aqueous acid, a process that is believed to involve the aryl cation instead of radical and is known to generate other nucleophilic addition side products in addition to the desired hydroxylation product.