Phenylpropanoid
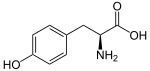
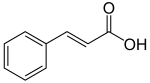
[1] Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis.
From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids.
Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds.
Conversion of these acids to their corresponding esters produces some of the volatile components of herb and flower fragrances, which serve many functions such as attracting pollinators.
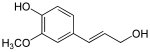
The monolignols are monomers that are polymerized to generate various forms of lignin and suberin, which are used as a structural component of plant cell walls.