Phosphaethynolate
[7] Similar studies were performed on derivatives of this structure and the results indicated that dimerisation to form a four-membered Li ring is favoured by this molecule.
[5] Ten years later, in 2002, Westerhausen et al. published the use of Becker's method to make a family of alkaline earth metal salts of PCO (see Scheme 2); this work involved the synthesis of the magnesium, calcium, strontium and barium bis-phosphaethynolates.
[5][8] Like the salts previously reported by Becker, the alkali-earth metal analogues were unstable to moisture and air and thus were required to be stored at low temperatures (around −20 °C) in dimethoxyethane solutions.
[9] The authors noted that this sodium salt could be handled in air as well as water without major decomposition; this emphasises the significance of the accompanying counter cation in stabilisation of PCO.
[6][9] Direct carbonylation was a method also employed by Goicoechea in 2013 in order to synthesis a phosphaethynolate anion stabilised by a potassium cation sequestered in 18-crown-6 (see Scheme 4).
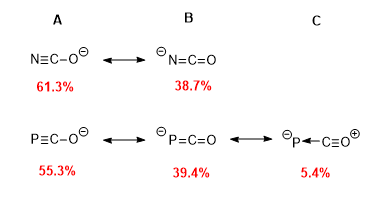
[11] The two dominant resonance forms of the phosphaethynolate anion localise negative charge on either the phosphorus or oxygen atoms meaning both are sites of nucleophilicity.
[12] The group's studies used a Re(I) complex and the analysis of its bonding parameters and electronic structure showed that the phosphaethynolate anion coordinated in a bent fashion.
[3] Initially when reacting the anion with triorganyl silicon compounds, it binds via the oxygen forming the kinetic oxyphosphaalkyne product.
[15][16] In 2014, Grutzmacher et al. reported that an imidazolium salt would react with the phosphaethynolate anion to produce a phosphinidine carbene adduct.
[15] The results of these investigations suggested that the lowest energy and therefore most likely pathway involves PCO acting as a Brønsted base initially deprotonating the acidic imidazolium cation to generate the intermediate phosphaketene, HPCO.
[1][15][17] The highly unstable protonated PCO remains hydrogen bonded to the newly produced N-heterocylic carbene prior to rearrangement and formation of the observed product.
[10] They found that the anion could react in a [2+2] fashion with a diphenyl ketene to produce the first isolatable example of a four-membered monoanionic phosphorus containing heterocycle.
[10] Cycloaddition reactions involving the phosphaethynolate anion have also been shown by Grutzmacher and co-workers to be a viable synthetic route to other heterocycles.
[1][18] A large part of the research involving PCO is now looking into utilising the anion as a synthetic building block to derive phosphorus containing analogues of small molecules.
[19] Generating acylphosphines in this manner is considered a much milder route than other current strategies that require multi-step syntheses involving toxic, volatile and pyrophoric reagents.
[7] In addition, NBO analysis highlights that the greatest electron delocalisation within the anions stems from the donation of an oxygen lone pair into the E−C π antibonding orbital.
[11] This is the result of a reduced difference in electronegativity between E and X thus neither atom offers a substantial advantage over the other in terms of providing ionic contributions to bonding.