Phosphate-buffered saline
Phosphate-buffered saline (PBS) is a buffer solution (pH ~ 7.4) commonly used in biological research.
The osmolarity and ion concentrations of the solutions are isotonic, meaning they match those of the human body.
[1] There are many different ways to prepare PBS solutions, common ones are Dulbecco's phosphate-buffered saline (DPBS)[2] and the Cold Spring Harbor protocol.
[4] If used in cell culturing, the solution can be dispensed into aliquots and sterilized by autoclaving or filtration.
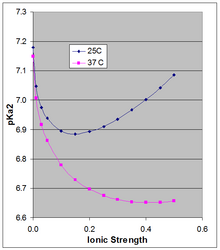
However the pKa is dependent on ionic strength and temperature, and as it shifts so will the pH of a solution based on that acid–base pair.
This applies strictly to the extrapolated thermodynamic pKa0 at infinite dilution, and as the figure shows, the temperature effect can be much larger at higher ionic strength.