Phosphofructokinase 2
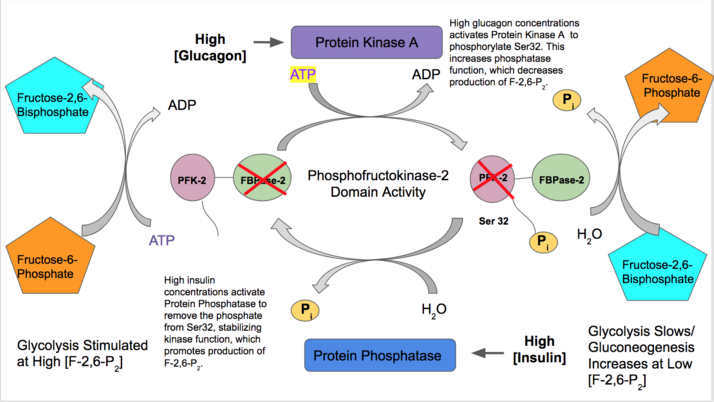
[1] Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways.
[1] Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs.
The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart.
[3] The family described here bears a resemblance to the ATP-driven phospho-fructokinases; however, they share little sequence similarity, although a few residues seem key to their interaction with fructose 6-phosphate.
[9] The PFK-2 domain appears to be closely related to the superfamily of mononucleotide binding proteins including adenylate cyclase.
[12][13] While this central catalytic core remains conserved in all forms of PFK-2, slight structural variations exist in isoforms as a result of different amino acid sequences or alternative splicing.
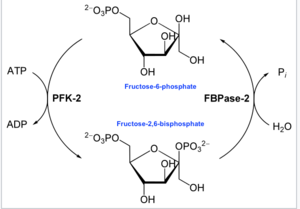
This enzyme's main function is to synthesize or degrade allosteric regulator Fru-2,6-P2 in response to glycolytic needs of the cell or organism, as depicted in the accompanying diagram.In enzymology, a 6-phosphofructo-2-kinase (EC 2.7.1.105) is an enzyme that catalyzes the chemical reaction: Thus, the kinase domain hydrolyzes ATP to phosphorylate the carbon-2 of fructose-6-phosphate, producing Fru-2,6-P2 and ADP.
[16] On the other hand, the phosphatase reaction is characteristic of the family of hydrolases, specifically those acting on phosphoric monoester bonds.
Phosphorylation of a specific residue may prompt a shift that stabilizes either kinase or phosphatase domain function.
[20] High levels of AMP or phosphate group signifies a low energy charge state and thus stimulates PFK2.
[14] Because these areas often contain phosphorylation sites, changes in amino acid composition or terminal length may result in vastly different enzyme kinetics and characteristics.
[1][14] Each variant differs in their primary tissue of expression, response to protein kinase regulation, and ratio of kinase/phosphatase domain activity.
[47] Because this enzyme family maintains rates of glycolysis and gluconeogenesis, it presents great potential for therapeutic action for control of metabolism particularly in diabetes and cancer cells.