Photosensitizer
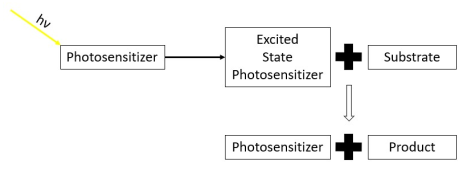
At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light.
[5] Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy.
This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation.
Photosensitizers experience varying levels of efficiency for intersystem crossing at different wavelengths of light based on the internal electronic structure of the molecule.
Photoacids increase in acidity upon absorbing light and thermally reassociate back into their original form upon relaxing.
[12][13] Currently, photosensitizers are studied for their contributions to fields such as energy harvesting, photoredox catalysis in synthetic chemistry, and cancer treatment.
Some notable organic photosensitizers which have been studied extensively include benzophenones, methylene blue, rose Bengal, flavins, pterins[23] and others.
Monatomic gaseous mercury (considered as the smallest possible cluster compound) is a photosensitizer catalyzing radical dehydrogenation.
Some key advantages to the use of quantum dots as photosensitizers includes their small, tunable band gap which allows for efficient transitions to the triplet state, and their insolubility in many solvents which allows for easy retrieval from a synthetic reaction mixture.
[26] Photodynamic therapy utilizes Type II photosensitizers to harvest light to degrade tumors or cancerous masses.
[11] The photodynamic process is predominantly a noninvasive technique wherein the photosensitizers are put inside a patient so that it may accumulate on the tumor or cancer.
When the photosensitizer reaches the tumor or cancer, wavelength specific light is shined on the outside of the patient's affected area.
This light (preferably near infrared frequency as this allows for the penetration of the skin without acute toxicity) excites the photosensitizer's electrons into the triplet state.
[16][14][31][32] Via the absorption of light, photosensitizers can utilize triplet state transfer to reduce small molecules, such as water, to generate Hydrogen gas.
[33][34] In the early 20th century, chemists observed that various aromatic hydrocarbons in the presence of oxygen could absorb wavelength specific light to generate a peroxide species.
These photosensitizers used in redox chemistry may be organic, organometallic, or nanomaterials depending on the physical and spectral properties required for the reaction.
[16][24] Photosensitizers that are readily incorporated into the external tissues can increase the rate at which reactive oxygen species are generated upon exposure to UV light (such as UV-containing sunlight).
Some photosensitizing agents, such as St. John's Wort, appear to increase the incidence of inflammatory skin conditions in animals and have been observed to slightly reduce the minimum tanning dose in humans.