Delta-aminolevulinic acid dehydratase
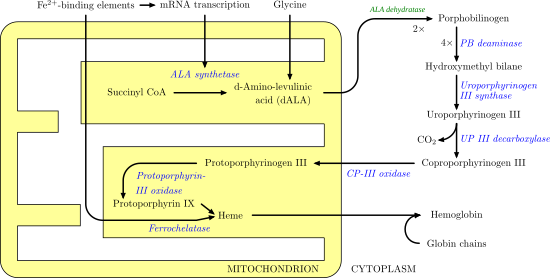
All natural tetrapyrroles, including hemes, chlorophylls and vitamin B12, share porphobilinogen as a common precursor.
The * represents a reorientation between two domains of each subunit that occurs in the dissociated state because it is sterically forbidden in the larger multimers.
As a nearly universal enzyme with a highly conserved active site, PBGS would not be a prime target for the development of antimicrobials and/or herbicides.
[8] Though it is not common to consider hydronium ions as allosteric regulators, in the case of PBGS, side chain protonation at locations other than the active site has been shown to affect the quaternary structure equilibrium, and thus to affect the rate of its catalyzed reaction as well.
Small molecule binding to this phylogenetically variable cavity has been proposed to stabilize 6mer* of the targeted PBGS and consequently inhibit activity.
A defect in the ALAD structural gene can cause increased sensitivity to lead poisoning and acute hepatic porphyria.
[14] All disease associated protein variants favor hexamer formation relative to the wild type human enzyme.