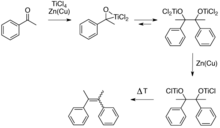
McMurry reaction
Related species have been developed involving the combination of TiCl3 or TiCl4 with various other reducing agents, including potassium, zinc, and magnesium.
The nature of low-valent titanium species formed is varied as the products formed by reduction of the precursor titanium halide complex will naturally depend upon both the solvent (most commonly THF or DME) and the reducing agent employed: typically, lithium aluminum hydride, zinc-copper couple, zinc dust, magnesium-mercury amalgam, magnesium, or alkali metals.
[4] Bogdanovic and Bolte identified the nature and mode of action of the active species in some classical McMurry systems,[5] and an overview of proposed reaction mechanisms has been published.
[4] The original publication by Mukaiyama demonstrated reductive coupling of ketones using reduced titanium reagents.
[6] McMurry and Fleming coupled retinal to give carotene using a mixture of titanium trichloride and lithium aluminium hydride.