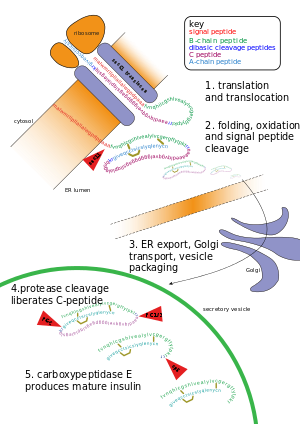
Post-translational modification
Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini.
[1] They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate.
[6] Sites that often undergo post-translational modification are those that have a functional group that can serve as a nucleophile in the reaction: the hydroxyl groups of serine, threonine, and tyrosine; the amine forms of lysine, arginine, and histidine; the thiolate anion of cysteine; the carboxylates of aspartate and glutamate; and the N- and C-termini.
[15] In 2011, statistics of each post-translational modification experimentally and putatively detected have been compiled using proteome-wide information from the Swiss-Prot database.
PTM information can be collected through experimental means or predicted from high-quality, manually curated data.