Potassium channel
They also regulate cellular processes such as the secretion of hormones (e.g., insulin release from beta-cells in the pancreas) so their malfunction can lead to diseases (such as diabetes).
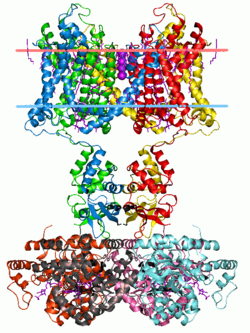
Potassium channels have a tetrameric structure in which four identical protein subunits associate to form a fourfold symmetric (C4) complex arranged around a central ion conducting pore (i.e., a homotetramer).
Alternatively four related but not identical protein subunits may associate to form heterotetrameric complexes with pseudo C4 symmetry.
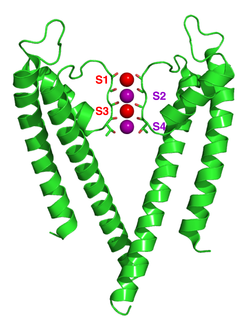
The four sets of electronegative carbonyl oxygen atoms are aligned toward the center of the filter pore and form a square antiprism similar to a water-solvating shell around each potassium binding site.
[60] This width appears to be maintained by hydrogen bonding and van der Waals forces within a sheet of aromatic amino acid residues surrounding the selectivity filter.
[55][61] The selectivity filter opens towards the extracellular solution, exposing four carbonyl oxygens in a glycine residue (Gly79 in KcsA).
[62][69] The prediction of an ion conduction mechanism in which the two doubly occupied states (S1, S3) and (S2, S4) play an essential role has been affirmed by both techniques.
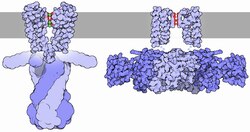
The presence of the cavity can be understood intuitively as one of the channel's mechanisms for overcoming the dielectric barrier, or repulsion by the low-dielectric membrane, by keeping the K+ ion in a watery, high-dielectric environment.
The flux of ions through the potassium channel pore is regulated by two related processes, termed gating and inactivation.
Generally, gating is thought to be mediated by additional structural domains which sense stimuli and in turn open the channel pore.
There are a number of structural models of C-type inactivated K+ channel filters,[81][82][83] although the precise mechanism remains unclear.
An example of one of these competitors is quaternary ammonium ions, which bind at the extracellular face[84][85] or central cavity of the channel.
[87] Barium ions can also block potassium channel currents,[88][89] by binding with high affinity within the selectivity filter.
[90][91][92][93] This tight binding is thought to underlie barium toxicity by inhibiting potassium channel activity in excitable cells.
[96][97] Roderick MacKinnon commissioned Birth of an Idea, a 5-foot (1.5 m) tall sculpture based on the KcsA potassium channel.