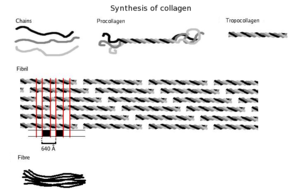
Collagen
Gelatin, which is used in food and industry, is collagen that was irreversibly hydrolyzed using heat, basic solutions, or weak acids.
Collagen contribution to the measure of cardiac performance summarily represents a continuous torsional force opposed to the fluid mechanics of blood pressure emitted from the heart.
The mass, distribution, age, and density of collagen all contribute to the compliance required to move blood back and forth.
[citation needed] As the skeleton forms the structure of the body, it is vital that it maintains its strength, even after breaks and injuries.
The triple helical structure prevents collagen from being broken down by enzymes, it enables adhesiveness of cells and it is important for the proper assembly of the extracellular matrix.
[16] Collagen has favorable properties for tissue regeneration, such as pore structure, permeability, hydrophilicity, and stability in vivo.
Collagen scaffolds also support deposition of cells, such as osteoblasts and fibroblasts, and once inserted, facilitate growth to proceed normally.
[18][19] These collagens may be derived from cow, horse, pig, or even human sources; and are sometimes used in combination with silicones, glycosaminoglycans, fibroblasts, growth factors and other substances.
[20] Collagen is one of the body's key natural resources and a component of skin tissue that can benefit all stages of wound healing.
[24] This kind of regular repetition and high glycine content is found in only a few other fibrous proteins, such as silk fibroin.
The relatively high content of proline and hydroxyproline rings, with their geometrically constrained carboxyl and (secondary) amino groups, along with the rich abundance of glycine, accounts for the tendency of the individual polypeptide strands to form left-handed helices spontaneously, without any intrachain hydrogen bonding.
Because glycine is the smallest amino acid with no side chain, it plays a unique role in fibrous structural proteins.
[24] This lower thermal stability means that gelatin derived from fish collagen is not suitable for many food and industrial applications.
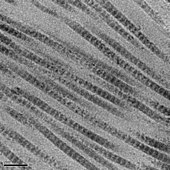
The tropocollagen subunits spontaneously self-assemble, with regularly staggered ends, into even larger arrays in the extracellular spaces of tissues.
[35] These later advances are particularly important to better understanding the way in which collagen structure affects cell–cell and cell–matrix communication and how tissues are constructed in growth and repair and changed in development and disease.
Collagen-related diseases most commonly arise from genetic defects or nutritional deficiencies that affect the biosynthesis, assembly, posttranslational modification, secretion, or other processes involved in normal collagen production.
[41] Osteogenesis imperfecta – Caused by a mutation in type 1 collagen, dominant autosomal disorder, results in weak bones and irregular connective tissue, some cases can be mild while others can be lethal.
[42] Chondrodysplasias – Skeletal disorder believed to be caused by a mutation in type 2 collagen, further research is being conducted to confirm this.
Patients present with protrusion of the brain tissue and degeneration of the retina; an individual who has family members with the disorder is at an increased risk of developing it themselves since there is a hereditary link.
Collagen has great tensile strength, and is the main component of fascia, cartilage, ligaments, tendons, bone and skin.
[48][49] Along with elastin and soft keratin, it is responsible for skin strength and elasticity, and its degradation leads to wrinkles that accompany aging.
[54] Limited tests have been done on the tensile strength of the collagen fiber, but generally it has been shown to have a lower Young's modulus compared to fibrils.
However, at the macro, tissue scale, the vast number of structures that collagen fibers and fibrils can be arranged into results in highly variable properties.
The oldest glue in the world, carbon-dated as more than 8,000 years old, was found to be collagen – used as a protective lining on rope baskets and embroidered fabrics, to hold utensils together, and in crisscross decorations on human skulls.
Animal glues are thermoplastic, softening again upon reheating, so they are still used in making musical instruments such as fine violins and guitars, which may have to be reopened for repairs – an application incompatible with tough, synthetic plastic adhesives, which are permanent.
Gelatin-resorcinol-formaldehyde glue (and with formaldehyde replaced by less-toxic pentanedial and ethanedial) has been used to repair experimental incisions in rabbit lungs.
Collagen supplements, derived from sources like fish and cattle, are marketed to improve skin, hair, and nails.
Studies show some skin benefits, but these supplements often contain other beneficial ingredients, making it unclear if collagen alone is effective.
Overall, the effectiveness of oral collagen supplements is not well-proven, and focusing on a healthy lifestyle and proven skincare methods like sun protection is recommended.
[33][81][82] As with its monomeric structure, several conflicting models propose either that the packing arrangement of collagen molecules is 'sheet-like', or is microfibrillar.