Prokaryotic cytoskeleton
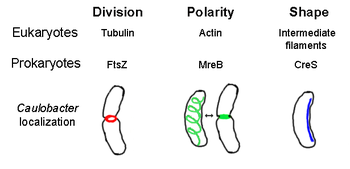
[7][8] FtsZ, the first identified prokaryotic cytoskeletal element, forms a filamentous ring structure located in the middle of the cell called the Z-ring that constricts during cell division, similar to the actin-myosin contractile ring in eukaryotes.
MreB assembles into a helical network of filamentous structures just under the cytoplasmic membrane, covering the whole length of the cell.
[14] ParM is a cytoskeletal element that possesses a similar structure to actin, although it behaves functionally like tubulin.
ParM affixes to ParR, a DNA-binding protein that specifically binds to 10 direct repeats in the parC region on the R1 plasmid.
[17] Lately an actin-like ParM homolog has been found in a gram-positive bacterium Bacillus thuringiensis, which assembles into a microtubule-like structure and is involved in plasmid segregation.
[18] Crenactin is an actin homologue unique to the archaeal kingdom Thermoproteota (formerly Crenarchaeota) that has been found in the orders Thermoproteales and Candidatus Korarchaeum.
[20] Crenactin has been well characterized in Pyryobaculum calidifontis (A3MWN5) and shown to have high specificity for ATP and GTP.
[22] Crescentin (encoded by creS gene) is an analogue of eukaryotic intermediate filaments (IFs).
The MinCDE helix occupies a pole and terminates in a filamentous structure called the E-ring made of MinE at the middle-most edge of the polar zone.
[24] The dynamic behavior of the Min proteins has been reconstituted in vitro using an artificial lipid bilayer as mimic for the cell membrane.
[25] Bactofilin (InterPro: IPR007607) is a β-helical cytoskeletal element that forms filaments throughout the cells of the rod-shaped proteobacterium Myxococcus xanthus.