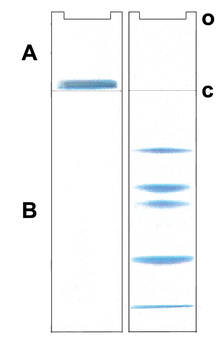
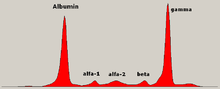
Gel electrophoresis of proteins
The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium, namely agarose or polyacrylamide.
Gel electrophoresis is often performed in combination with electroblotting or immunoblotting to give additional information about a specific protein.
[1] SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, describes a collection of related techniques to separate proteins according to their electrophoretic mobility (a function of the molecular weight of a polypeptide chain) while in the denatured (unfolded) state.
In most proteins, the binding of SDS to the polypeptide chain imparts an even distribution of charge per unit mass, thereby resulting in a fractionation by approximate size during electrophoresis.
[4] BN-PAGE is a native PAGE technique, where the Coomassie brilliant blue dye provides the necessary charges to the protein complexes for the electrophoretic separation.
[citation needed] CN-PAGE (commonly referred to as Native PAGE) separates acidic water-soluble and membrane proteins in a polyacrylamide gradient gel.
[7] The folded protein complexes of interest separate cleanly and predictably without the risk of denaturation due to the specific properties of the polyacrylamide gel, electrophoresis buffer solution, electrophoretic equipment and standardized parameters used.
[9] Most protein separations are performed using a "discontinuous" (or DISC) buffer system that significantly enhances the sharpness of the bands within the gel.
The resolving gel typically has a much smaller pore size, which leads to a sieving effect that now determines the electrophoretic mobility of the proteins.
[12][13] As voltage is applied, the anions (and negatively charged sample molecules) migrate toward the positive electrode (anode) in the lower chamber, the leading ion is Cl− ( high mobility and high concentration); glycinate is the trailing ion (low mobility and low concentration).
[14] The boundary moves through a pore gradient and the protein stack gradually disperses due to a frictional resistance increase of the gel matrix.
For a complete protein unstacking the polyacrylamide-gel concentration must exceed 16% T. The two-gel system of "Laemmli" is a simple gradient gel.
Silver staining is a sensitive procedure to detect trace amounts of proteins in gels, but can also visualize nucleic acid or polysaccharides.
Abnormal bands (spikes) are seen in monoclonal gammopathy of undetermined significance and multiple myeloma, and are useful in the diagnosis of these conditions.