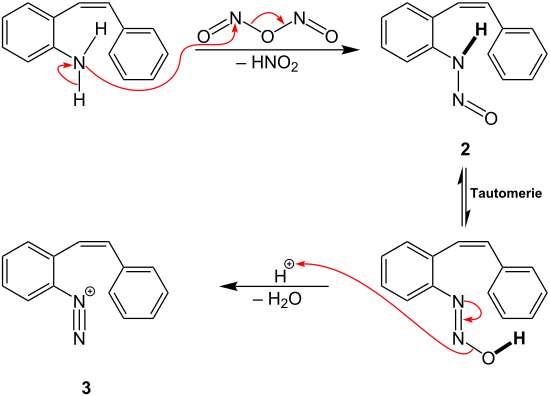
Pschorr cyclization
It describes the intramolecular substitution of aromatic compounds via aryldiazonium salts as intermediates and is catalyzed by copper.
A nitroso group is introduced as new substituent, producing under the release of nitrous acid intermediate 2.
The aryl radical thus formed reacts via ring closure to the intermediate stage 4.
Finally, rearomatization takes place using again the copper catalyst and phenanthrene is formed.
The Pschorr cyclization has a relatively good atom economy, since essentially only nitrogen is produced as a waste material.