Pyridine
Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity.
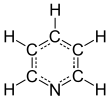
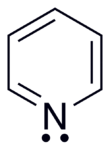
In contrast to benzene, the electron density is not evenly distributed over the ring, reflecting the negative inductive effect of the nitrogen atom.
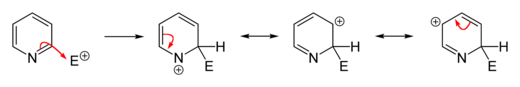
As a result, the lone pair does not contribute to the aromatic system but importantly influences the chemical properties of pyridine, as it easily supports bond formation via an electrophilic attack.
[26] However, because of the separation of the lone pair from the aromatic ring system, the nitrogen atom cannot exhibit a positive mesomeric effect.
Impure pyridine was undoubtedly prepared by early alchemists by heating animal bones and other organic matter,[27] but the earliest documented reference is attributed to the Scottish scientist Thomas Anderson.
[29] Among other substances, he separated from the oil a colorless liquid with unpleasant odor, from which he isolated pure pyridine two years later.
Owing to its flammability, Anderson named the new substance pyridine, after Greek: πῦρ (pyr) meaning fire.
Wilhelm Körner (1869)[32] and James Dewar (1871)[33][34] suggested that, in analogy between quinoline and naphthalene, the structure of pyridine is derived from benzene by substituting one C–H unit with a nitrogen atom.
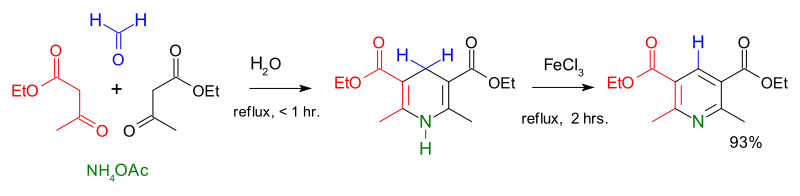
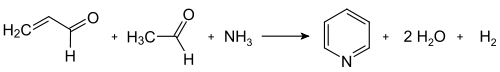
[41] The Hantzsch pyridine synthesis typically uses a 2:1:1 mixture of a β-keto acid (often acetoacetate), an aldehyde (often formaldehyde), and ammonia or its salt as the nitrogen donor.
[42] The contemporary methods of pyridine production had a low yield, and the increasing demand for the new compound urged to search for more efficient routes.
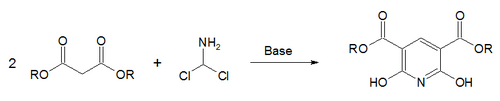
A breakthrough came in 1924 when the Russian chemist Aleksei Chichibabin invented a pyridine synthesis reaction, which was based on inexpensive reagents.
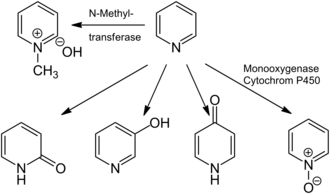
[50] Traces of pyridine can be found in Beaufort cheese,[51] vaginal secretions,[52] black tea,[53] saliva of those suffering from gingivitis,[54] and sunflower honey.
The reaction of pyridine with bromomethyl ketones gives the related pyridinium salt, wherein the methylene group is highly acidic.
[83] Correspondingly pyridine is more prone to nucleophilic substitution, as evidenced by the ease of metalation by strong organometallic bases.
The reaction with many Lewis acids results in the addition to the nitrogen atom of pyridine, which is similar to the reactivity of tertiary amines.
This lone pair does not overlap with the aromatic π-system ring, consequently pyridine is basic, having chemical properties similar to those of tertiary amines.
[88] Owing to the decreased electron density in the aromatic system, electrophilic substitutions are suppressed in pyridine and its derivatives.
Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
These reactions include substitutions with elimination of a hydride ion and elimination-additions with formation of an intermediate aryne configuration, and usually proceed at the 2- or 4-position.
The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
For this purpose, pyridine derivatives can be eliminated with good leaving groups using strong bases such as sodium and potassium tert-butoxide.
The subsequent addition of a nucleophile to the triple bond has low selectivity, and the result is a mixture of the two possible adducts.
Radical dimerization of pyridine with elemental sodium or Raney nickel selectively yields 4,4'-bipyridine,[98] or 2,2'-bipyridine,[99] which are important precursor reagents in the chemical industry.
The Zincke reaction is used for the selective introduction of radicals in pyridinium compounds (it has no relation to the chemical element zinc).
[102] Selective synthesis of 1,4-dihydropyridine is achieved in the presence of organometallic complexes of magnesium and zinc,[103] and (Δ3,4)-tetrahydropyridine is obtained by electrochemical reduction of pyridine.
The η6 coordination mode, as occurs in η6 benzene complexes, is observed only in sterically encumbered derivatives that block the nitrogen center.
[24] Cetylpyridinium and laurylpyridinium, which can be produced from pyridine with a Zincke reaction, are used as antiseptic in oral and dental care products.
[119] Pyridine depresses the nervous system giving symptoms similar to intoxication with vapor concentrations of above 3600 ppm posing a greater health risk.
[2] The effects may have a delayed onset of several hours and include dizziness, headache, lack of coordination, nausea, salivation, and loss of appetite.
An allocation of positions by letter of the Greek alphabet (α-γ) and the substitution pattern nomenclature common for homoaromatic systems (ortho, meta, para) are used sometimes.