Quinuclidone
Quinuclidones are a class of bicyclic organic compounds with chemical formula C7H11NO with two structural isomers for the base skeleton 3-quinuclidone and 2-quinuclidone.
This behaviour is predicted by Bredt's Rule, and formal amide group resembles in fact an amine, as evidenced by the ease of salt formation.
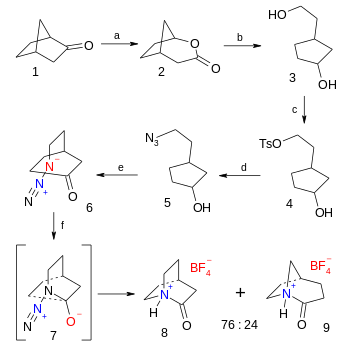
The organic synthesis of the tetrafluoroborate salt of 2-quinuclidone is a six-step affair starting from norcamphor the final step being an azide - ketone Schmidt reaction (38% yield):[5] This compound rapidly reacts with water to the corresponding amino acid with a chemical half-life of 15 seconds.
X-ray diffraction shows pyramidalization on the nitrogen atom (59° compared to 0 for reference dimethylformamide) and torsion around the carbon-nitrogen bond to an extent of 91°.
Further analysis via the extended kinetic method allows for the determination of the proton affinity and gas phase basicity of 2-quinuclidonium.