R-loop
The term "R-loop" was given to reflect the similarity of these structures to D-loops; the "R" in this case represents the involvement of an RNA moiety.
Sharp showed that protein coding adenovirus genes contained DNA sequences that were not present in the mature mRNA.
[12] In 1994, R-loops were demonstrated to be present in vivo through analysis of plasmids isolated from E. coli mutants carrying mutations in topoisomerase.
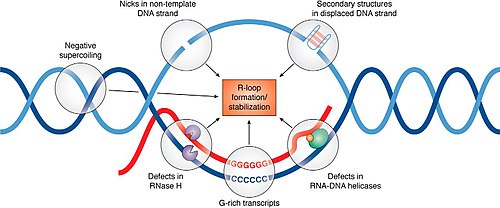
RNaseH enzymes are the primary proteins responsible for the dissolution of R-loops, acting to degrade the RNA moiety in order to allow the two complementary DNA strands to anneal.
Senataxin is one helicase that can move along ssRNA, and appears to be necessary for preventing R-loop formation at transcription pause sites.
[17] The third enzyme class capable of removing R-loops are branchpoint translocases such as FANCM, SMARCAL1 and ZRANB3 in humans or RecG in bacteria.
R-loop formation is a key step in immunoglobulin class switching, a process that allows activated B cells to modulate antibody production.
Bonnet et al. (2017)[26] speculated that the function of introns in maintaining genetic stability may explain their evolutionary maintenance at certain locations, particularly in highly expressed genes.