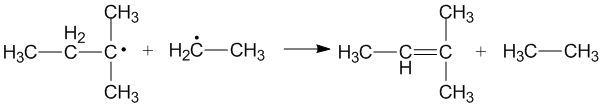Radical disproportionation
Due to the reactive nature of radical molecules, disproportionation proceeds rapidly and requires little to no activation energy.
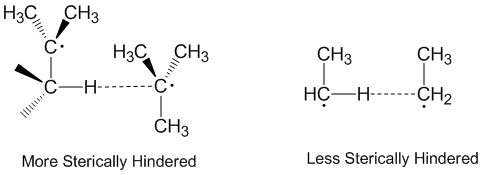
[2] Molecules that are more sterically hindered require arrangements that are more linear, and thus react more slowly.
[6] Alkyl radical disproportionation has been studied extensively in scientific literature.
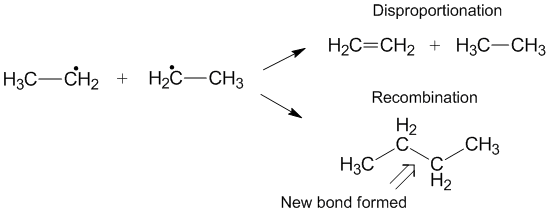
Similar to disproportionation, the recombination reaction is exothermic and requires little to no activation energy.
Thus disproportionation is weakly affected by the kinetic isotope effect with kH/kD = 1.20 ± 0.15 for ethylene.
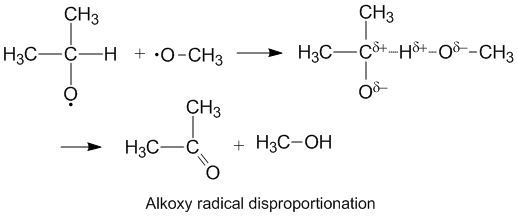
The oxygen has a partial negative charge which removes electron density from the donor carbon atom thereby facilitating hydrogen abstraction.
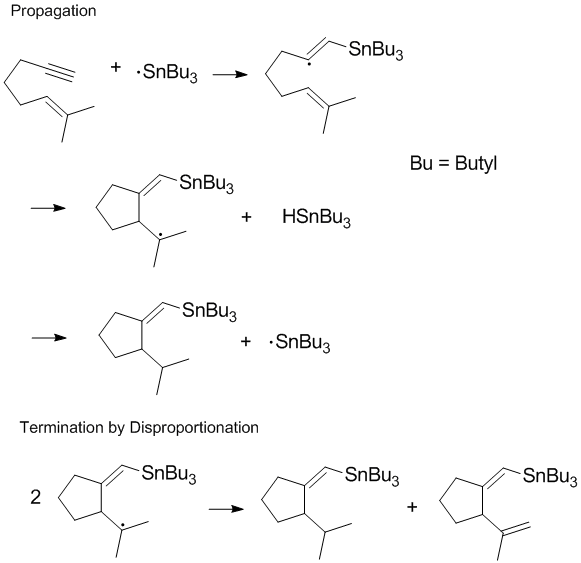
In some reactions (such as the one shown below) one or both of the termination pathways can be hindered by steric or solvent effects.
[10] During living free radical polymerization, termination pathways for a growing polymer chain are removed.