Phase (matter)
More precisely, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform.
[1][2]: 86 [3]: 3 Examples of physical properties include density, index of refraction, magnetization and chemical composition.
These two usages are not commensurate with the formal definition given above and the intended meaning must be determined in part from the context in which the term is used.
Distinct phases may be described as different states of matter such as gas, liquid, solid, plasma or Bose–Einstein condensate.
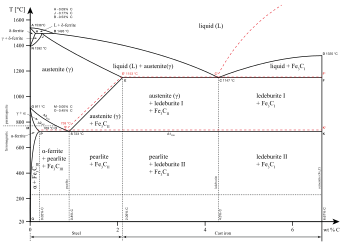
As shown in the diagram for iron alloys, several phases exist for both the solid and liquid states.
Emulsions and colloids are examples of immiscible phase pair combinations that do not physically separate.
Water in a closed jar with an air space over it forms a two-phase system.
At equilibrium, evaporation and condensation processes exactly balance and there is no net change in the volume of either phase.
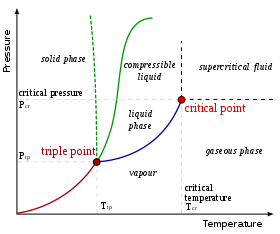
As the temperature and pressure approach the critical point, the properties of the liquid and gas become progressively more similar.
Increasing the pressure drives the water into the higher density phase, which causes melting.
Consider a test apparatus consisting of a closed and well-insulated cylinder equipped with a piston.
If the piston is slowly lowered, the system will trace a curve of increasing temperature and pressure within the gas region of the phase diagram.
Although this region may be very thin, it can have significant and easily observable effects, such as causing a liquid to exhibit surface tension.
In terms of modeling, describing, or understanding the behavior of a particular system, it may be efficacious to treat the interfacial region as a separate phase.
A single material may have several distinct solid states capable of forming separate phases.
When a substance undergoes a phase transition (changes from one state of matter to another) it usually either takes up or releases energy.