Radiocarbon dating
When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of 14C it contains begins to decrease as the 14C undergoes radioactive decay.
As a result, beginning in the late 19th century, there was a noticeable drop in the proportion of 14C as the carbon dioxide generated from burning fossil fuels began to accumulate in the atmosphere.
In 1939, Martin Kamen and Samuel Ruben of the Radiation Laboratory at Berkeley began experiments to determine if any of the elements common in organic matter had isotopes with half-lives long enough to be of value in biomedical research.
[1] At some time during World War II, Willard Libby, who was then at Berkeley, learned of Korff's research and conceived the idea that it might be possible to use radiocarbon for dating.
[5][6] Libby and several collaborators proceeded to experiment with methane collected from sewage works in Baltimore, and after isotopically enriching their samples they were able to demonstrate that they contained 14C.
[5] The variation in the 14C/12C ratio in different parts of the carbon exchange reservoir means that a straightforward calculation of the age of a sample based on the amount of 14C it contains will often give an incorrect result.
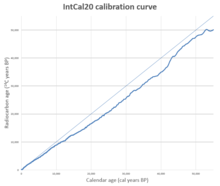
[39][41] Equipped with the results of carbon-dating the tree rings, it became possible to construct calibration curves designed to correct the errors caused by the variation over time in the 14C/12C ratio.
Correcting for isotopic fractionation, as is done for all radiocarbon dates to allow comparison between results from different parts of the biosphere, gives an apparent age of about 400 years for ocean surface water.
The atmospheric 14C/12C ratio is lower in the southern hemisphere, with an apparent additional age of about 40 years for radiocarbon results from the south as compared to the north.
The process takes about a month and requires a sample about ten times as large as would be needed otherwise, but it allows more precise measurement of the 14C/12C ratio in old material and extends the maximum age that can be reliably reported.
[62] For decades after Libby performed the first radiocarbon dating experiments, the only way to measure the 14C in a sample was to detect the radioactive decay of individual carbon atoms.
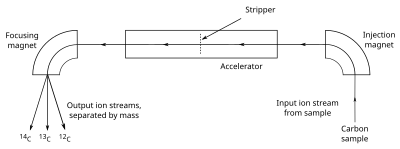
[63] In the late 1970s an alternative approach became available: directly counting the number of 14C and 12C atoms in a given sample, via accelerator mass spectrometry, usually referred to as AMS.
[59] Any interposing material would have interfered with the detection of radioactivity, since the beta particles emitted by decaying 14C are so weak that half are stopped by a 0.01 mm thickness of aluminium.
[60] Libby's method was soon superseded by gas proportional counters, which were less affected by bomb carbon (the additional 14C created by nuclear weapons testing).
The counters work by detecting flashes of light caused by the beta particles emitted by 14C as they interact with a fluorescing agent added to the benzene.
Libby's value for the half-life is used to maintain consistency with early radiocarbon testing results; calibration curves include a correction for this, so the accuracy of final reported calendar ages is assured.
[64] Radiocarbon dates are generally presented with a range of one standard deviation (usually represented by the Greek letter sigma as 1σ) on either side of the mean.
The results varied widely (though consistently with a normal distribution of errors in the measurements), and included multiple date ranges (of 1σ confidence) that did not overlap with each other.
If 1% of the benzene in a modern reference sample accidentally evaporates, scintillation counting will give a radiocarbon age that is too young by about 80 years.
[84] The improvements to these curves are based on new data gathered from tree rings, varves, coral, plant macrofossils, speleothems, and foraminifera.
[91] The technique is not restricted to tree rings; for example, a stratified tephra sequence in New Zealand, believed to predate human colonization of the islands, has been dated to 1314 AD ± 12 years by wiggle-matching.
[94] In addition, an article in Radiocarbon in 2014 about radiocarbon date reporting conventions recommends that information should be provided about sample treatment, including the sample material, pretreatment methods, and quality control measurements; that the citation to the software used for calibration should specify the version number and any options or models used; and that the calibrated date should be given with the associated probabilities for each range.
[101] Recent advances in field collection techniques also allow the radiocarbon dating of methane and carbon dioxide, which are important greenhouse gases.
This led to estimates that the trees were between 24,000 and 19,000 years old,[104] and hence this was taken to be the date of the last advance of the Wisconsin glaciation before its final retreat marked the end of the Pleistocene in North America.
There was initial resistance to these results on the part of Ernst Antevs, the palaeobotanist who had worked on the Scandinavian varve series, but his objections were eventually discounted by other geologists.
[104] The Two Creeks radiocarbon dates are now regarded as a key result in developing the modern understanding of North American glaciation at the end of the Pleistocene.
[106] In 1947, scrolls were discovered in caves near the Dead Sea that proved to contain writing in Hebrew and Aramaic, most of which are thought to have been produced by the Essenes, a small Jewish sect.
[110] In the words of anthropologist R. E. Taylor, "14C data made a world prehistory possible by contributing a time scale that transcends local, regional and continental boundaries".
It provides more accurate dating within sites than previous methods, which usually derived either from stratigraphy or from typologies (e.g. of stone tools or pottery); it also allows comparison and synchronization of events across great distances.
The advent of radiocarbon dating may even have led to better field methods in archaeology since better data recording leads to a firmer association of objects with the samples to be tested.



C in each reservoir [ 5 ] [ note 7 ]
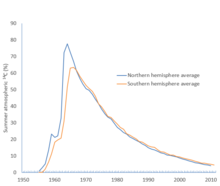
C for the northern and southern hemispheres, showing percentage excess above pre-bomb levels. The Partial Test Ban Treaty went into effect on 10 October 1963. [ 38 ]


C is now most commonly done with an accelerator mass spectrometer