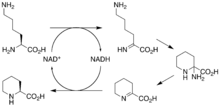Sirolimus
[2] Sirolimus (Fyarro), as protein-bound particles, is indicated for the treatment of adults with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumour (PEComa).
[3][17] In the EU, sirolimus, as Rapamune, is indicated for the prophylaxis of organ rejection in adults at low to moderate immunological risk receiving a renal transplant[5] and, as Hyftor, is indicated for the treatment of facial angiofibroma associated with tuberous sclerosis complex.
However, on 7 October 2008, the FDA approved safety labeling revisions for sirolimus to warn of the risk for decreased renal function associated with its use.
[18][19] In 2009, the FDA notified healthcare professionals that a clinical trial conducted by Wyeth showed an increased mortality in stable liver transplant patients after switching from a calcineurin inhibitor-based immunosuppressive regimen to sirolimus.
[12] In May 2015, the FDA approved sirolimus to treat lymphangioleiomyomatosis (LAM), a rare, progressive lung disease that primarily affects women of childbearing age.
[22] LAM involves lung tissue infiltration with smooth muscle-like cells with mutations of the tuberous sclerosis complex gene (TSC2).
Loss of TSC2 gene function activates the mTOR signaling pathway, resulting in the release of lymphangiogenic growth factors.
Treatment with sirolimus can decrease pain and the fullness of vascular malformations, improve coagulation levels, and slow the growth of abnormal lymphatic vessels.
Hence, sirolimus is ideal for "proliferative" vascular tumors through the control of tissue overgrowth disorders caused by inappropriate activation of the PI3K/AKT/mTOR pathway as an antiproliferative agent.
A retrospective review of English-language medical publications reporting on topical sirolimus treatment of facial angiofibromas found sixteen separate studies with positive patient outcomes after using the drug.
[29] The most common adverse reactions (≥30% occurrence, leading to a 5% treatment discontinuation rate) observed with sirolimus in clinical studies of organ rejection prophylaxis in individuals with kidney transplants include: peripheral edema, hypercholesterolemia, abdominal pain, headache, nausea, diarrhea, pain, constipation, hypertriglyceridemia, hypertension, increased creatinine, fever, urinary tract infection, anemia, arthralgia, and thrombocytopenia.
[2] The most common adverse reactions (≥20% occurrence, leading to an 11% treatment discontinuation rate) observed with sirolimus in clinical studies for the treatment of lymphangioleiomyomatosis are: peripheral edema, hypercholesterolemia, abdominal pain, headache, nausea, diarrhea, chest pain, stomatitis, nasopharyngitis, acne, upper respiratory tract infection, dizziness, and myalgia.
[2][45] Individuals taking sirolimus are at increased risk of experiencing impaired or delayed wound healing, particularly if they have a body mass index in excess of 30 kg/m2 (classified as obese).
However, mTOR is now the widely accepted name, since Tor was first discovered via genetic and molecular studies of sirolimus-resistant mutants of Saccharomyces cerevisiae that identified FKBP12, Tor1, and Tor2 as the targets of sirolimus and provided robust support that the FKBP12-sirolimus complex binds to and inhibits Tor1 and Tor2.
[8] The biosynthesis of the rapamycin core is accomplished by a type I polyketide synthase (PKS) in conjunction with a nonribosomal peptide synthetase (NRPS).
The domains responsible for the biosynthesis of the linear polyketide of rapamycin are organized into three multienzymes, RapA, RapB, and RapC, which contain a total of 14 modules (figure 1).
[50] The gene rapL has been established to code for a NAD+-dependent lysine cycloamidase, which converts L-lysine to L-pipecolic acid (figure 4) for incorporation at the end of the polyketide.
In addition, genes rapI, rapJ, rapM, rapN, rapO, and rapQ have been identified as coding for tailoring enzymes that modify the macrocyclic core to give rapamycin (figure 3).
The starting unit is then modified by a series of Claisen condensations with malonyl or methylmalonyl substrates, which are attached to an acyl carrier protein (ACP) and extend the polyketide by two carbons each.
After each successive condensation, the growing polyketide is further modified according to enzymatic domains that are present to reduce and dehydrate it, thereby introducing the diversity of functionalities observed in rapamycin (figure 1).
Akt signalling promotes cell survival in Akt-positive lymphomas and acts to prevent the cytotoxic effects of chemotherapy drugs, such as doxorubicin or cyclophosphamide.
[61][62][63][64][65] Sirolimus also shows promise in treating tuberous sclerosis complex (TSC), a congenital disorder that predisposes those afflicted to benign tumor growth in the brain, heart, kidneys, skin, and other organs.
[68] Sirolimus has potential for widespread use as a longevity-promoting drug, with evidence pointing to its ability to prevent age-associated decline of cognitive and physical health.
In 2014, researchers at Novartis showed that a related compound, everolimus, increased elderly patients' immune response on an intermittent dose.
[75] When applied as a topical preparation, researchers showed that rapamycin can regenerate collagen and reverse clinical signs of aging in elderly patients.
[citation needed] Rapamycin has been proposed as a treatment for severe acute respiratory syndrome coronavirus 2 insofar as its immunosuppressive effects could prevent or reduce the cytokine storm seen in very serious cases of COVID-19.
Treatment often consists of removal of the affected tissue via excision, laser ablation or sclerotherapy, but the rate of recurrence can be high and surgery can have complications.
Sirolimus has shown evidence of being an effective treatment in alleviating symptoms and reducing the size of the malformation by way of altering the mTOR pathway in lymphangiogenesis.
[81] Due to its immunosuppressant activity, Rapamycin has been assessed as prophylaxis or treatment agent of Graft-versus-host disease (GVHD), a complication of hematopoietic stem cell transplantation.
[citation needed] A number of veterinary medicine teaching hospitals are participating in a long-term clinical study examining the effect of rapamycin on the longevity of dogs.